Polynorepinephrine modified magnetic particle and preparation method and application thereof
A technology of noradrenaline and magnetic particles, applied in the preparation of test samples, chemical instruments and methods, and other chemical processes, to achieve good adsorption and elution, mild conditions, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of polyNE-MNPs includes the following steps:
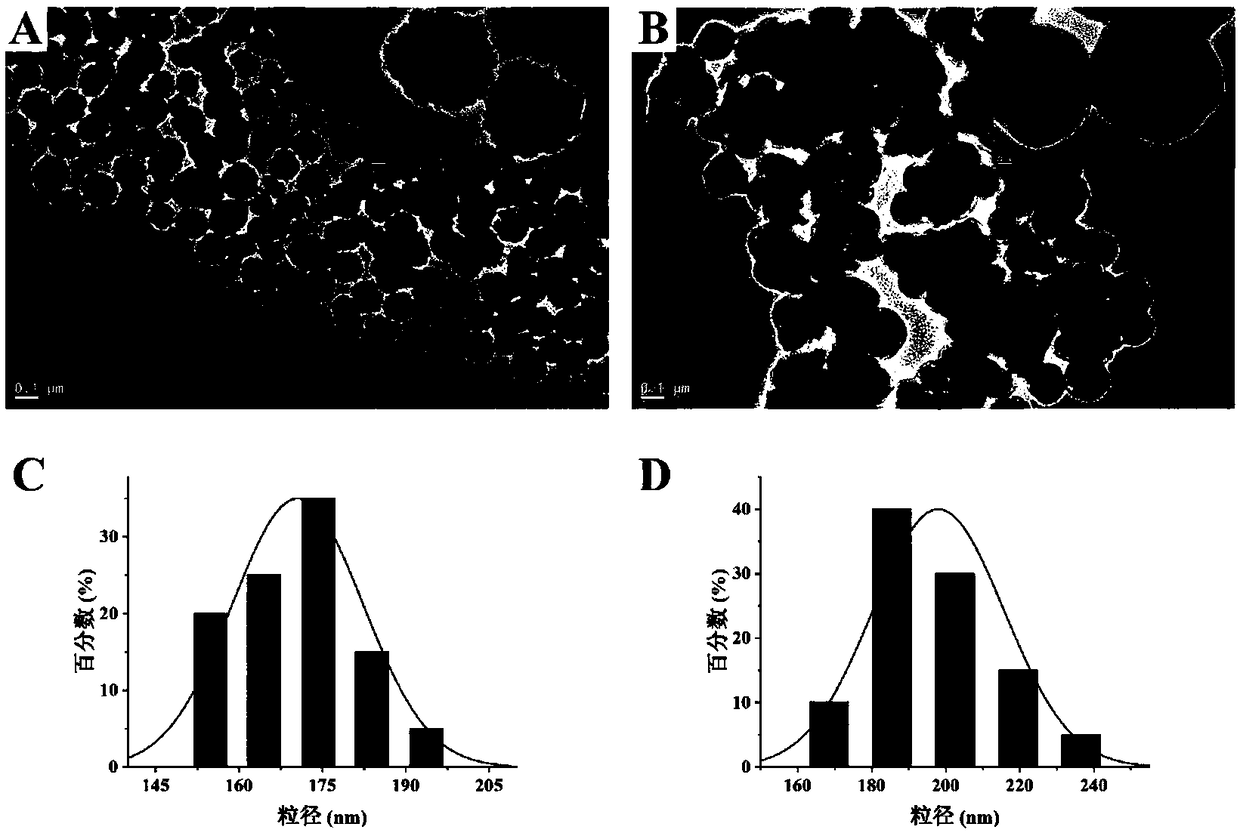
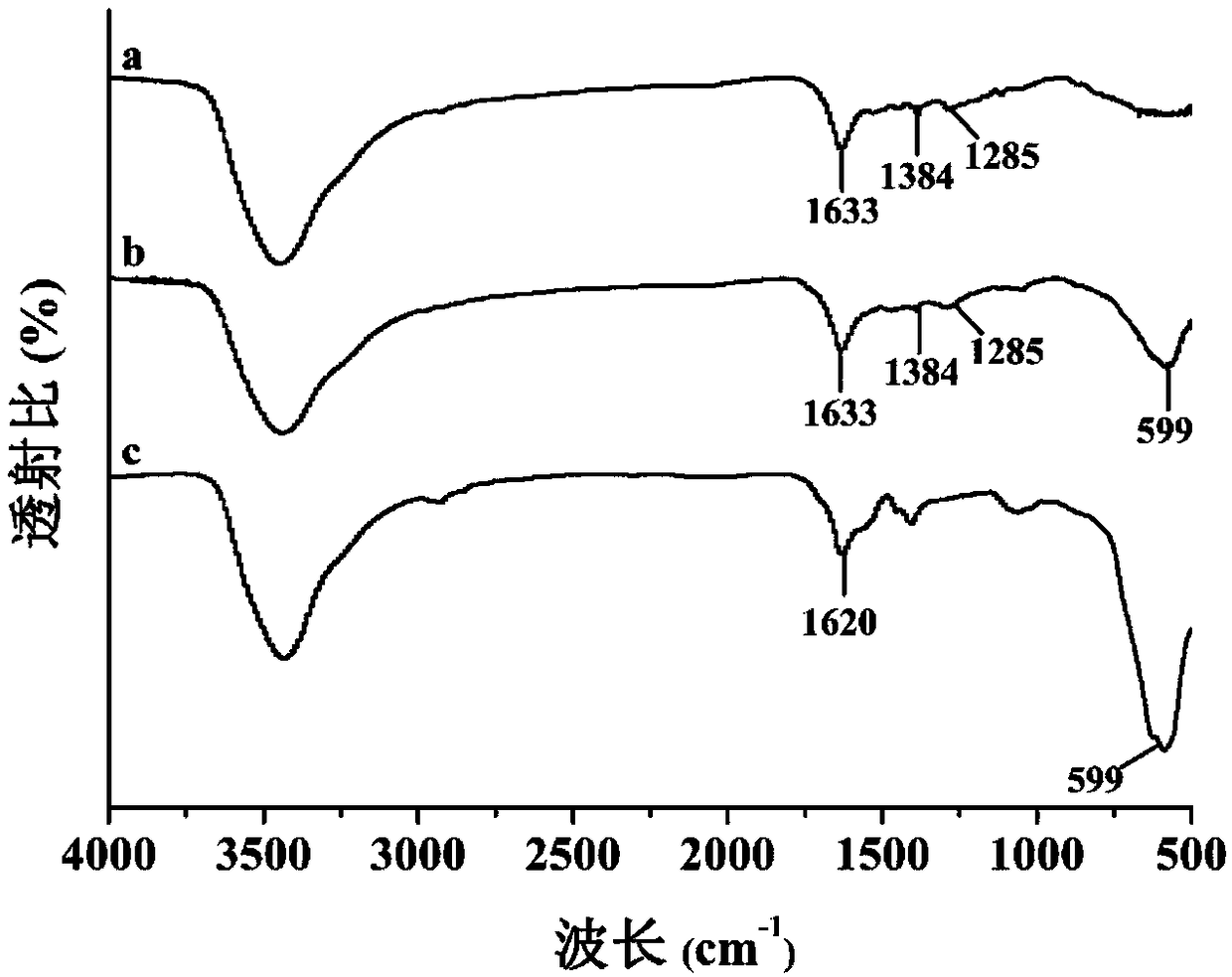
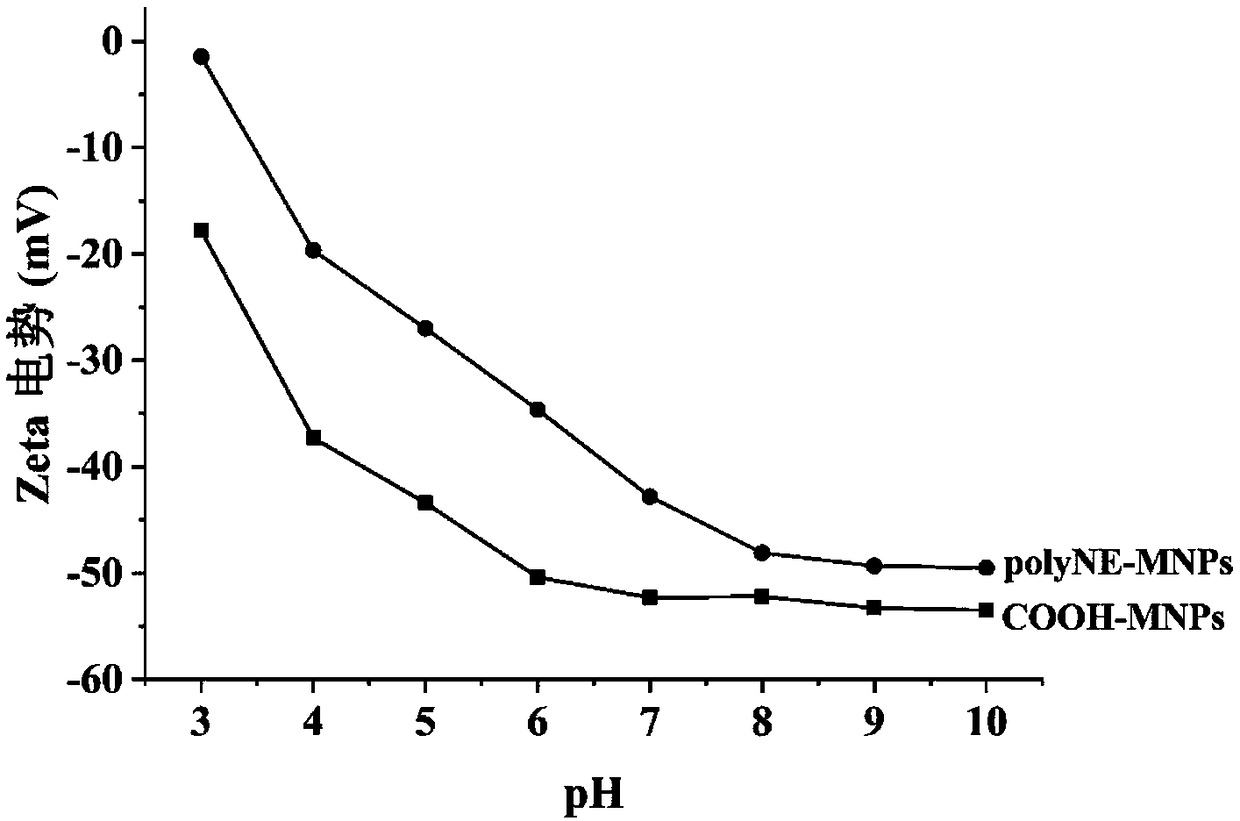
[0036] (1) Preparation of COOH-MNPs: 4mM FeCl 3 ·6H 2 O was added to 30mL of diethylene glycol and 10mL of ethylene glycol for ultrasonic dissolution, then 3g of anhydrous sodium acetate and 3g of sodium acrylate were added, and stirred in a constant temperature water bath at 70°C at a speed of 500r / min for 1h to form a homogeneous solution. Pour it into a Teflon high-pressure reactor, put it in an oven, heat up to 200°C, react for 10 hours, cool to room temperature, separate the product under an external magnetic field and wash it with ethanol and deionized water for 3 times, and dry it at 60°C Uniformly dispersed COOH-MNPs were obtained.
[0037] (2) 30 mg of norepinephrine was dissolved in Tris-HCl aqueous solution (10 mL, 50 mM, pH 8.5) to prepare a norepinephrine solution. Then, 25 mg COOH-MNPs were weighed and dispersed in the norepinephrine solution, and stirred at 40 °C for 12 h. The obtained pol...
Embodiment 2
[0045] The preparation of polyNE-MNPs includes the following steps:
[0046] (1) Preparation of COOH-MNPs: 5mM FeCl 3 ·6H 2 O was added to 20mL of diethylene glycol and 20mL of ethylene glycol for ultrasonic-assisted dissolution, then 6g of anhydrous sodium acetate and 3g of sodium acrylate were added, and stirred in a constant temperature water bath at 50°C at a speed of 300r / min for 1h to form a homogeneous solution. Pour it into a Teflon autoclave, put it in an oven, raise the temperature to 180°C, react for 15 hours, cool to room temperature, separate the product under an external magnetic field and wash it three times with de-ethanol and deionized water successively, and dry at 60°C Dry to obtain uniformly dispersed COOH-MNPs.
[0047] (2) 25 mg of norepinephrine was dissolved in Tris-HCl aqueous solution (10 mL, 30 mM, pH 7.5) to prepare a norepinephrine solution. Then, 25 mg COOH-MNPs were weighed and dispersed in the norepinephrine solution, and stirred at 50 °C for...
Embodiment 3
[0055] The preparation of polyNE-MNPs includes the following steps:
[0056] (1) Preparation of COOH-MNPs: 6mM FeCl 3 ·6H 2 O was added to 32mL of diethylene glycol and 8mL of ethylene glycol for ultrasonic-assisted dissolution, then 9g of anhydrous sodium acetate and 3g of sodium acrylate were added, and stirred in a constant temperature water bath at 60°C at a speed of 400r / min for 1h to form a homogeneous solution. Pour it into a Teflon autoclave, put it in an oven, raise the temperature to 190°C, react for 20 hours, cool to room temperature, separate the product under an external magnetic field and wash it three times with de-ethanol and deionized water successively, and dry at 60°C Dry to obtain uniformly dispersed COOH-MNPs.
[0057] (2) 50 mg of norepinephrine was dissolved in Tris-HCl aqueous solution (10 mL, 70 mM, pH 9.5) to prepare a norepinephrine solution. Then, 25 mg COOH-MNPs were weighed and dispersed in the norepinephrine solution, and stirred at 25 °C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com