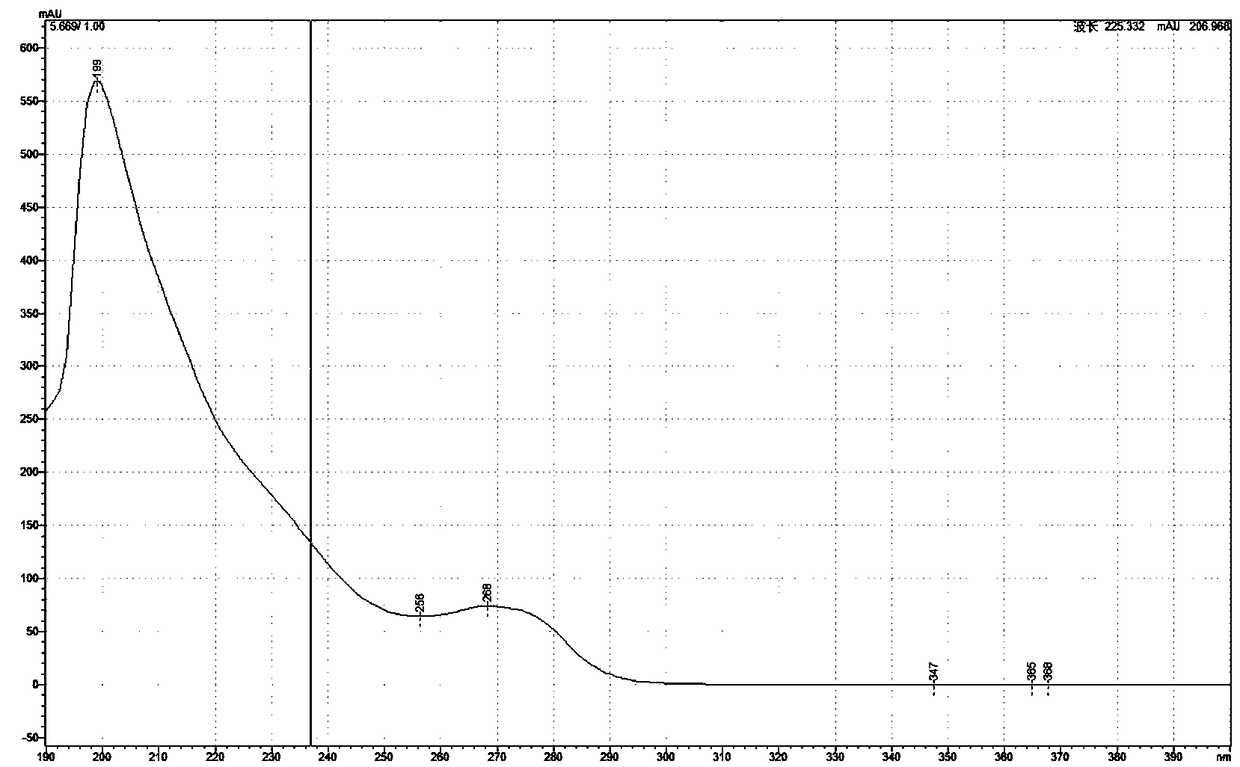
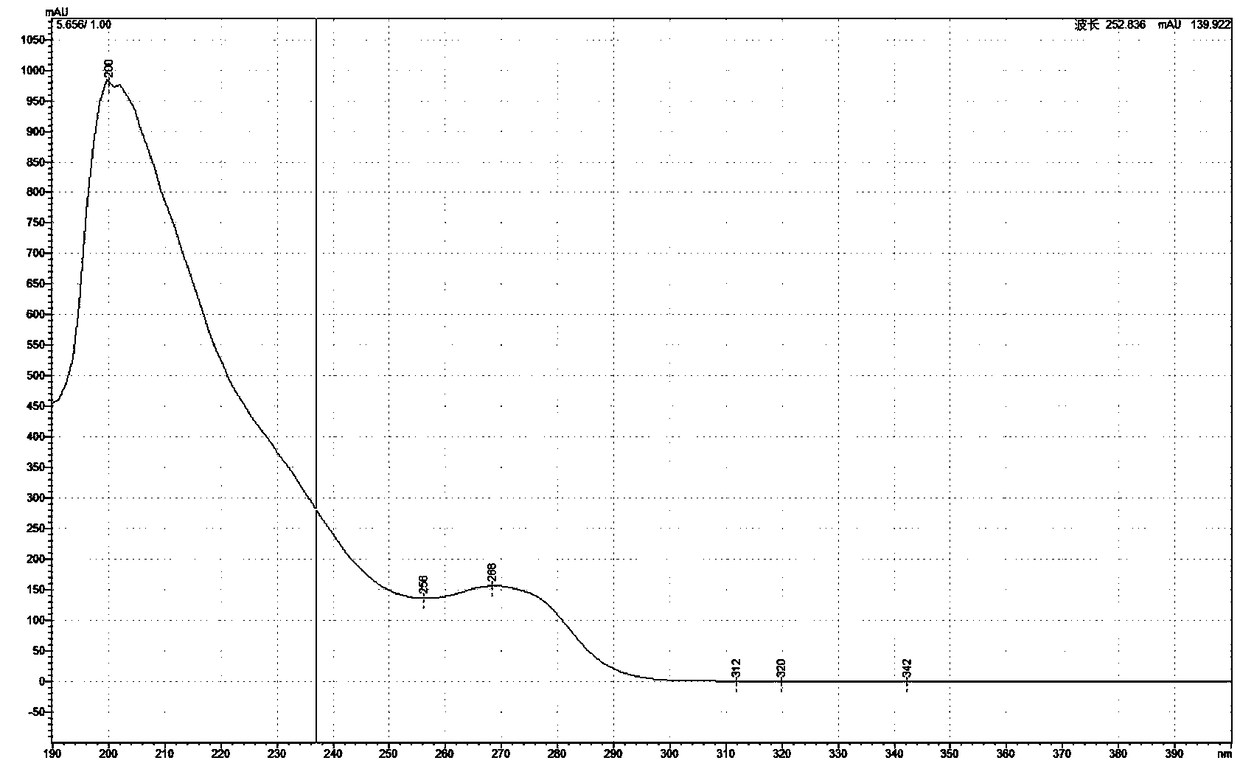
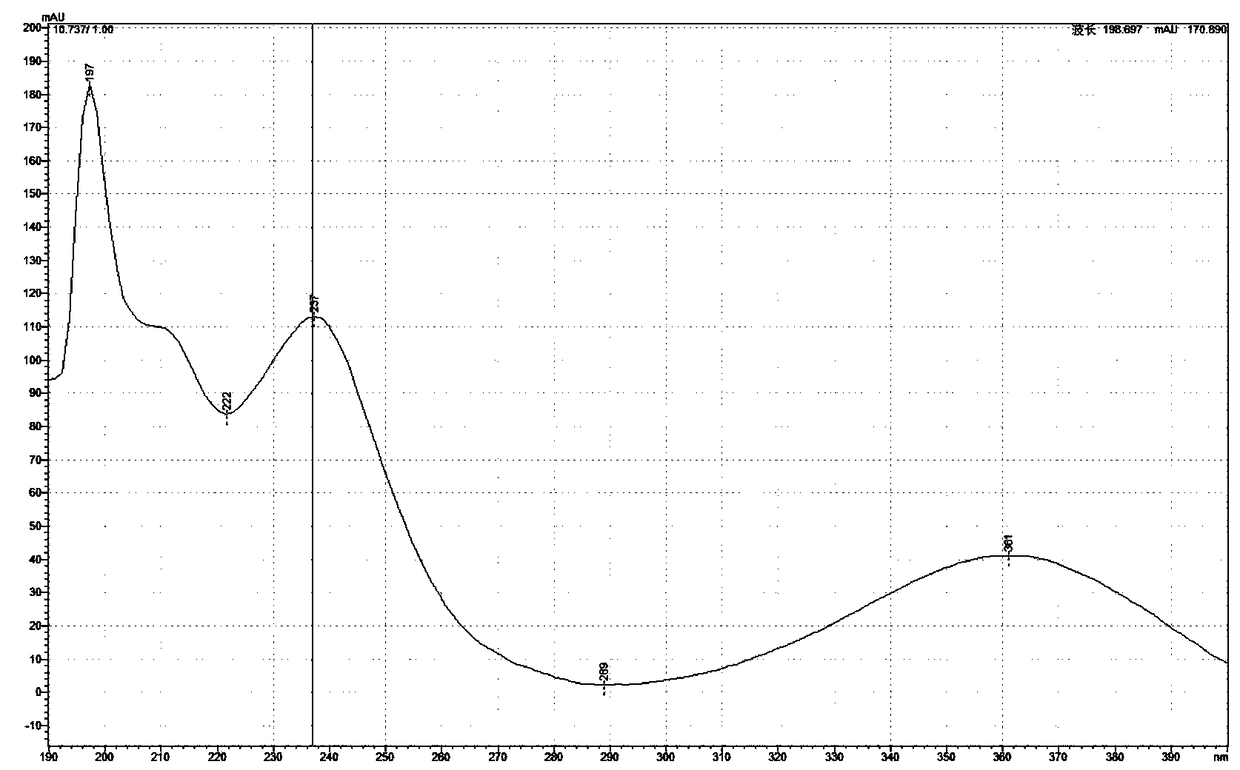
Method for dehydro-aromatization of dihydropyridine compound and application to drug detection
A technology for dehydroaromatization and dihydropyridine, which is applied in the fields of organic chemistry, drug combination, material testing products, etc., and can solve the problems of cumbersome, complicated, and many by-products in post-processing.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1: Dehydroaromatization of dihydropyridine compounds
[0048] (1) Dihydropyridine compounds (usually 200mg amount of feeding) are placed in the reaction flask, and acid-containing aqueous solution (hydrochloric acid; the amount of dihydropyridine compounds in every 100ml acid-containing aqueous solution is 1.2g; the acid concentration is 0.5 mol / L), stirring and dissolving;
[0049] (2) Add nickel (elemental nickel; per 100 parts by weight of dihydropyridine compounds, the amount of nickel added is 3 parts by weight as nickel; add in the form of 40 mesh fine particles) in the reaction flask, stir at room temperature to make the reaction Complete (6 hours);
[0050] (3) Add alkali (sodium hydroxide; 1.2M) to adjust the pH to 8.0, extract three times with dichloromethane (each with the same volume as the aqueous phase), combine the organic layers, wash with saturated sodium chloride, and collect the organic layers;
Embodiment 2
[0056] Embodiment 2: Dehydroaromatization of dihydropyridine compounds
[0057] (1) Dihydropyridine compounds (usually 200mg amount of feeding) are placed in the reaction flask, and acid-containing aqueous solution (hydrochloric acid; the amount of dihydropyridine compounds in every 100ml acid-containing aqueous solution is 1g; the acid concentration is 0.7mol / L), stirring and dissolving;
[0058] (2) Add nickel (elemental nickel; per 100 parts by weight of dihydropyridine compounds, the amount of nickel added is 4 parts by weight as nickel; add in the form of 30 mesh fine particles) in the reaction flask, stir at room temperature to make the reaction Complete (10 hours);
[0059] (3) Add alkali (sodium hydroxide; 2M) to adjust the pH value to 8.5, extract three times with dichloromethane (each with the same volume as the aqueous phase), combine the organic layers, wash with saturated sodium chloride, and collect the organic layers;
[0060] (4) Add anhydrous sodium sulfa...
Embodiment 3
[0065] Example 3: Dehydroaromatization of dihydropyridines
[0066] (1) Dihydropyridine compounds (usually 200mg amount of feeding) are placed in the reaction flask, and acid-containing aqueous solution (hydrochloric acid; the amount of dihydropyridine compounds in every 100ml acid-containing aqueous solution is 2g; the acid concentration is 0.3mol / L), stirring and dissolving;
[0067] (2) Add nickel (elemental nickel; per 100 parts by weight of dihydropyridine compounds, the amount of nickel added is 2.5 parts by weight as nickel; add in the form of 60 mesh fine particles), stir at room temperature to make the reaction Complete (4 hours);
[0068] (3) Add alkali (sodium hydroxide; 1M) to adjust the pH value to 7.5, extract three times with dichloromethane (each with the same volume as the aqueous phase), combine the organic layers, wash with saturated sodium chloride, and collect the organic layers;
[0069] (4) Add anhydrous sodium sulfate to dry (10 hours), filter, con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com