Cellulose-based camptothecin prodrug and preparation method thereof
A camptothecin and cellulose technology, which is applied to the field of cellulose-based camptothecin prodrugs and their preparation, can solve the problems of difficulty in ensuring selective and controllable drug release, poor selectivity, and low drug loading, and achieves structural Controllable, increase upload capacity, enhance the effect of internalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Preparation of a class of cellulose-based camptothecin prodrugs
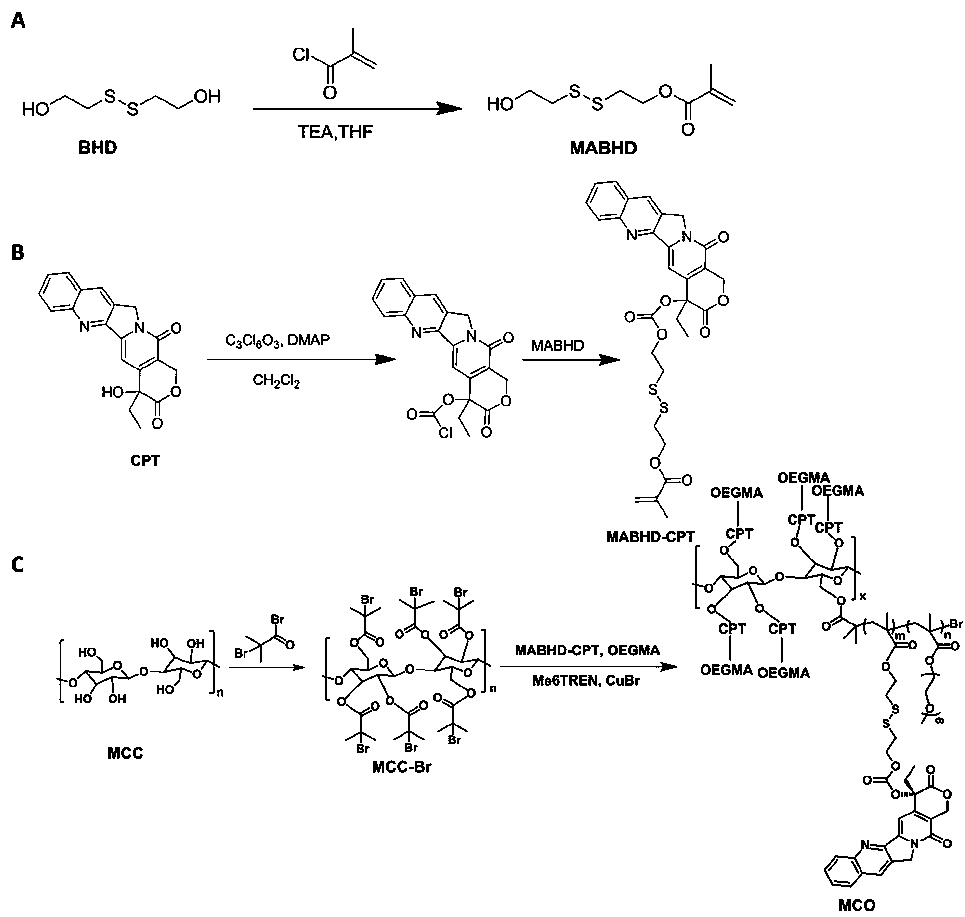
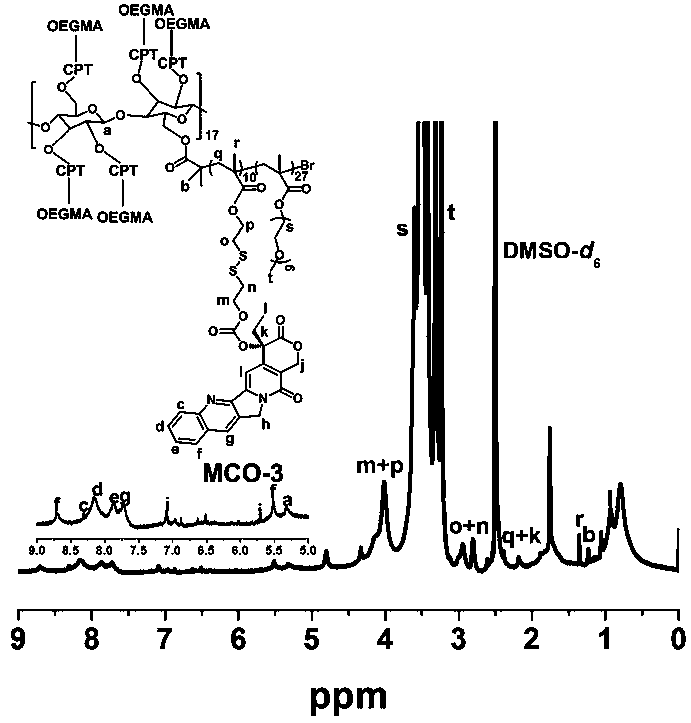
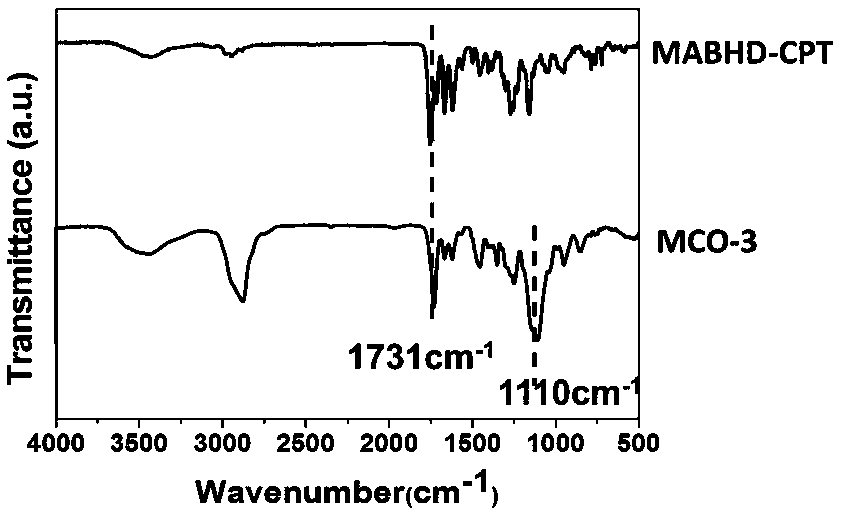
[0030] The schematic diagram of the total synthesis of a class of cellulose-based camptothecin prodrugs is shown as figure 1 As shown, it mainly includes the following steps:
[0031] (1) The preparation of intermediate MABHD containing disulfide bonds includes the following steps: in an ice bath and argon atmosphere, dissolve BHD (5g, 32.4mmoL) in 50mL THF, and then TEA (2.6mL, 18.9mmoL) ) Slowly add the above BHD solution in THF; Dissolve methacryloyl chloride (1.8mL, 18.2mmoL) in 10mL anhydrous THF, then add the methacryloyl solution dissolved in anhydrous THF slowly with stirring to In the above reaction system, the reaction was carried out at 0° C. for 0.5 h, and then heated to room temperature for 24 h. The column was purified to obtain the intermediate MABHD containing disulfide bonds.
[0032] (2) Preparation of MABHD-CPT, a CPT precursor containing disulfide bonds, includes the following step...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com