Synthetic method of benzindole derivative
A technology for benzoindole derivatives and synthesis methods, which is applied in the field of synthesis of benzoindole derivatives, can solve problems such as single catalyst, harsh reaction conditions, etc., and achieves strong substrate applicability, stable raw materials, and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
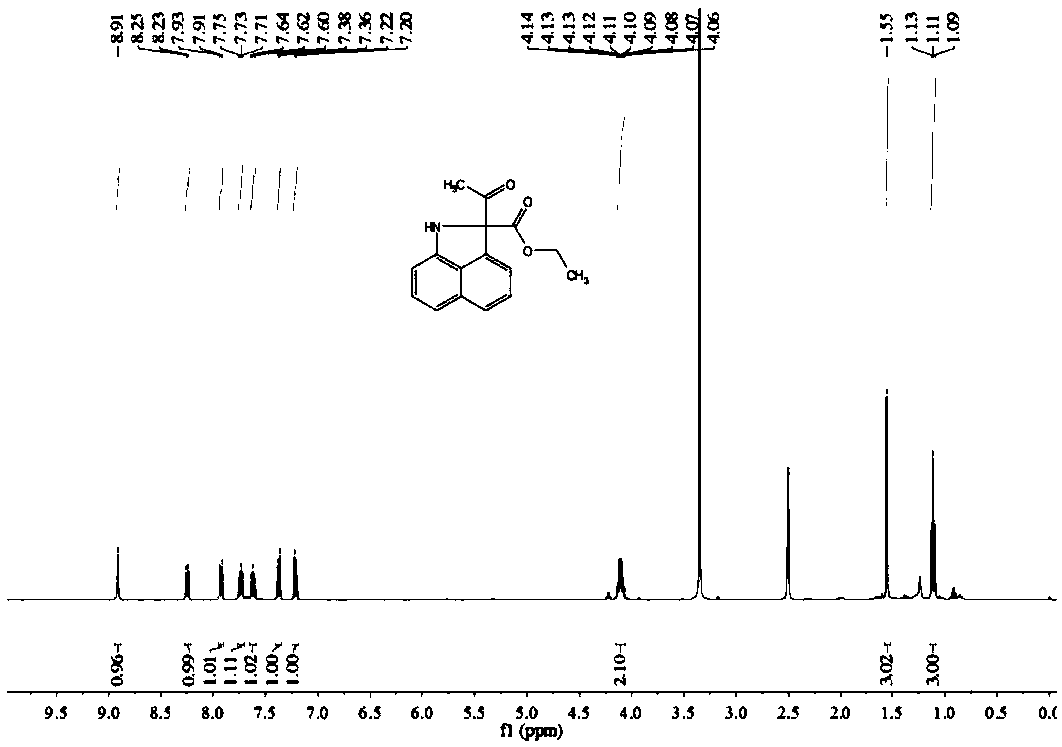
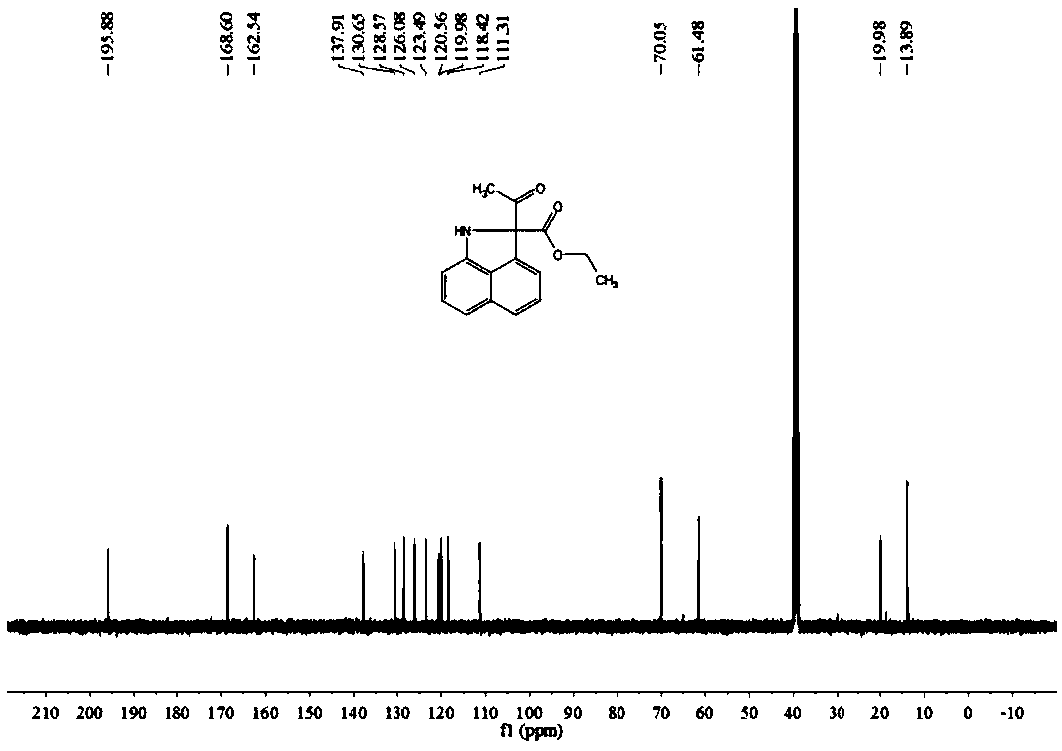
[0063] Preparation of Ethyl 2-acetyl-1,2-dihydrobenzo[c,d]indole-2-carboxylate
[0064] Dissolve 0.1 mmol of naphthylamine in 2 mL of H 2 In the Shrek tube of O, add 0.2mmol ethyl diazoacetoacetate, add 0.3mL good solvent ethanol to promote dissolution, and add 0.005mmol [RuCl 2 (p-cymene)] 2 As a catalyst, 0.025mmol cesium acetate was used as an additive, heated and stirred at 65°C for 16 hours, and the progress of the reaction was monitored by TLC. After the reaction was complete, it was extracted three times with 5mL of dichloromethane, the combined organic phase was concentrated, and separated by silica gel column chromatography. Obtain 19.2mg yellow oily compound, productive rate is 76%, and the product structural formula of gained is as follows:
[0065]
[0066] Such as figure 1 with figure 2 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ=8.91(s,1H),8.24(d,J=8.2Hz,1H),7.92(d,J=8.1Hz,1H),7.73(t,J=7.5Hz,1H),7.62(t, J=7.6Hz, 1H), 7.37(d, J=8.6Hz...
Embodiment 2
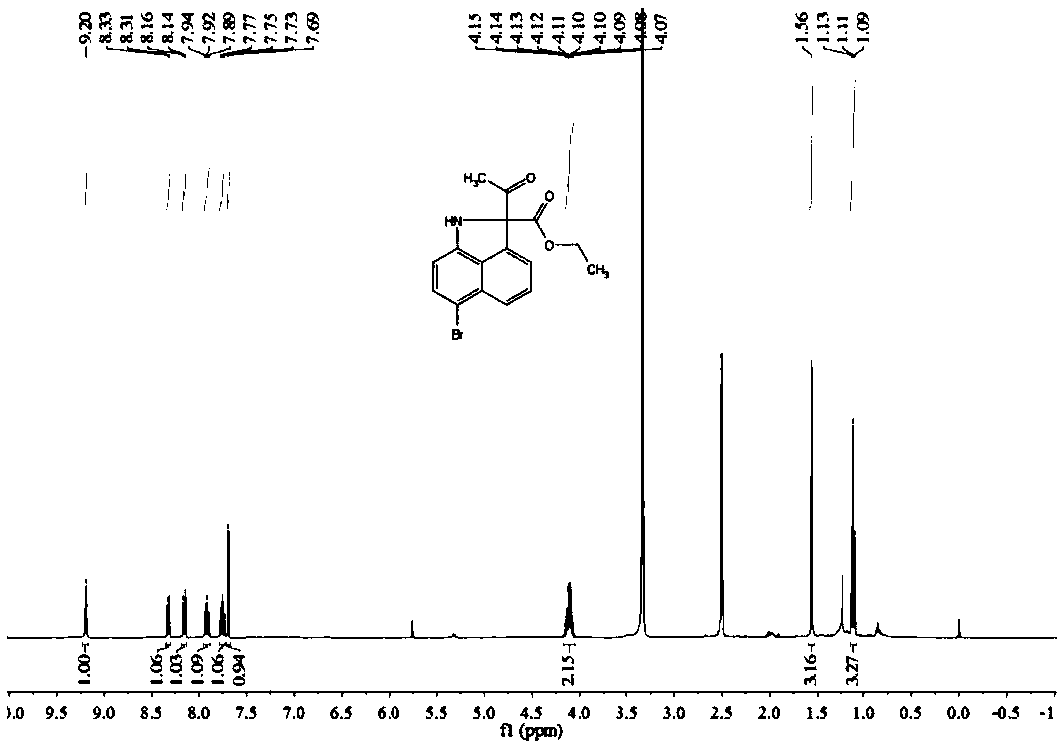
[0068] Preparation of Ethyl 2-acetyl-6-bromo-1,2-dihydrobenzo[c,d]indole-2-carboxylate
[0069] Dissolve 0.1 mmol of 4-bromo-1-naphthylamine in 2 mL of H 2 In the Shrek tube of O, add 0.2mmol ethyl diazoacetoacetate, add 0.3mL good solvent ethanol to promote dissolution, and add 0.005mmol [RuCl 2 (p-cymene)] 2 As a catalyst, 0.025mmol cesium acetate was used as an additive, heated and stirred at 65°C for 16 hours, and the progress of the reaction was monitored by TLC. After the reaction was complete, it was extracted three times with 5mL of dichloromethane, the combined organic phase was concentrated, and separated by silica gel column chromatography. Obtain 19.5mg yellow oily compound, productive rate is 59%, and the product structural formula of gained is as follows:
[0070]
[0071] Such as image 3 with Figure 4 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ=9.20(s,1H),8.32(d,J=7.7Hz,1H),8.15(d,J=8.3Hz,1H),7.92(t,J=8.3Hz,1H),7.75(t, J=8.1Hz, 1H...
Embodiment 3
[0073] Preparation of Ethyl 2-acetyl-6-amino-1,2-dihydrobenzo[c,d]indole-2-carboxylate
[0074] Dissolve 0.1 mmol of 4-amino-1-naphthylamine in 2 mL of H 2 In the Shrek tube of O, add 0.2mmol ethyl diazoacetoacetate, add 0.3mL good solvent ethanol to promote dissolution, and add 0.005mmol [RuCl 2 (p-cymene)] 2 As a catalyst, 0.025mmol cesium acetate was used as an additive, heated and stirred at 65°C for 16 hours, and the progress of the reaction was monitored by TLC. After the reaction was complete, it was extracted three times with 5mL of dichloromethane, the combined organic phase was concentrated, and separated by silica gel column chromatography. Obtain 17.7mg yellow oily compound, productive rate is 47%, and the product structural formula of gained is as follows:
[0075]
[0076] Such as Figure 5 with Image 6 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ=8.62(s,1H),7.37(dd,J=8.3,5.5Hz,2H),7.28(t,J=7.8Hz,1H),7.20(s,1H),6.91–6.88(m,1H ),5.85(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com