Blue-green fluorescent sandwich-type manganese coordination polymer, preparation method and application thereof to positive ion detection
A coordination polymer, blue-green fluorescence technology, applied in the field of sandwich-type manganese coordination polymers, can solve the problems of difficult fluorescent materials and applications in the detection field, non-fluorescence and other problems, and achieve the effect of simple operation and high thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 complex of the present invention
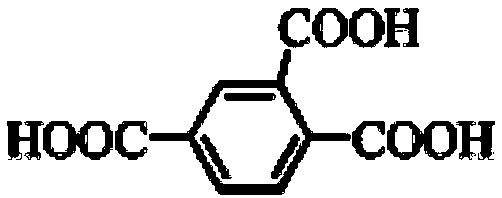
[0033] Weigh raw materials according to the following specific mass or volume: H 3 tma(0.05mmol), bpeb(0.025mmol), MnCl 2 4H 2 O (0.075mmol), HCl (30uL, 1.7mol / L), CH 3 CN (3 mL), water (7 mL).
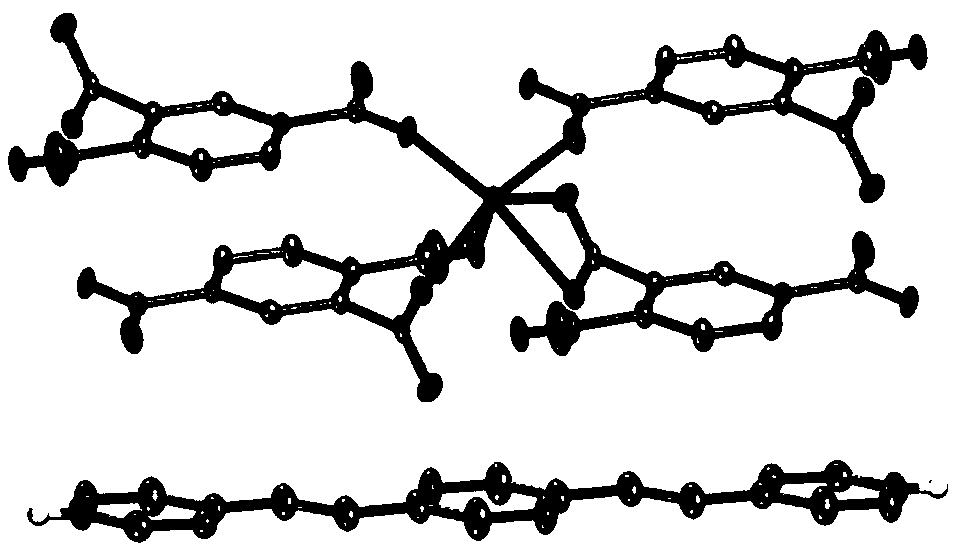
[0034] Placed in a 50mL glass beaker, the mixture was stirred for 0.5 hours (h), then transferred into a 25mL reactor, reacted at 140°C for two days, then cooled to room temperature naturally, and a block crystal was observed, which was filtered out from the mother liquor, CH 3 CN, wash with water, dry naturally.
[0035] Carry out powder diffraction test with Shimadzu XRD-6100 type X-ray diffractometer, the peak of test spectrum and the peak energy of the spectrum simulated by mercury software can be well matched, indicating that this crystal is the target product, and the sample purity is higher ( See Figure 4 ). Under the condition of air atmosphere, thermogravimetric analysis shows that the obtained...
Embodiment 2
[0040] The preparation of embodiment 2 complexes of the present invention
[0041] Weigh raw materials according to the following specific mass or volume: H 3 tma(0.05mmol), bpeb(0.025mmol), MnCl 2 4H 2 O (0.075mmol), HCl (20uL, 1.7mol / L), CH 3 CN (3 mL), water (7 mL).
[0042] Placed in a 50mL glass beaker, the mixture was stirred for 1.5 hours (h), then transferred into a 25mL reactor, reacted at 130°C for three days, then cooled to room temperature naturally, and a block crystal was observed, which was filtered out from the mother liquor, CH 3 CN, wash with water, dry naturally.
[0043] The product was characterized by X-ray powder diffraction, and the data obtained were similar to those in Example 1. Illustrate that the crystal structure that makes with embodiment 2 does not change and product is purer (see Figure 4 ).
[0044] This embodiment is repeated many times, and according to actual production, {[Mn 2 (tma) 2 ](H 2 bpeb)} n The quality of 12.4 ~ 12.7m...
Embodiment 3
[0045] The preparation of embodiment 3 complexes of the present invention
[0046] Weigh raw materials according to the following specific mass or volume: H 3 tma(0.05mmol), bpeb(0.025mmol), MnCl 2 4H 2 O (0.075mmol), HCl (40uL, 1.7mol / L), CH 3 CN (3 mL), water (7 mL).
[0047] Place in a 50mL glass beaker, stir the mixture for 1h, then transfer it into a 25mL reactor, react at 150°C for four days, then cool to room temperature naturally, observe yellow crystals, which are the target product, filter it out from the mother liquor, and then use CH 3 CN, wash with water, dry naturally.
[0048] The product was characterized by X-ray powder diffraction, and the data obtained were similar to those in Example 1. Illustrate that the crystal structure that makes with embodiment 3 does not change and product is purer (see Figure 4 ).
[0049] This embodiment is repeated many times, and according to actual production, {[Mn 2 (tma) 2 ](H 2 bpeb)} n The mass of 11.9 ~ 12.6mg,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com