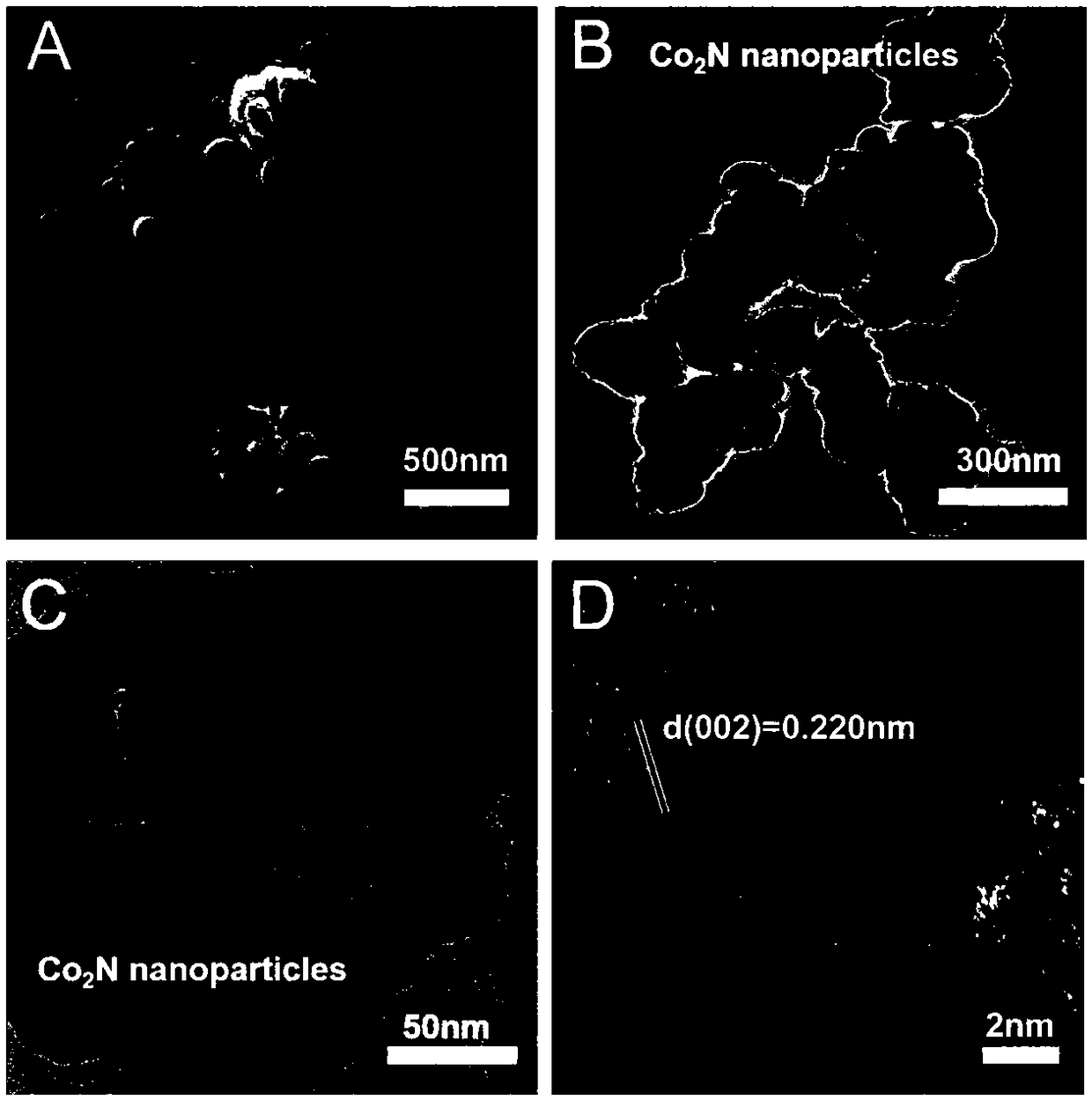
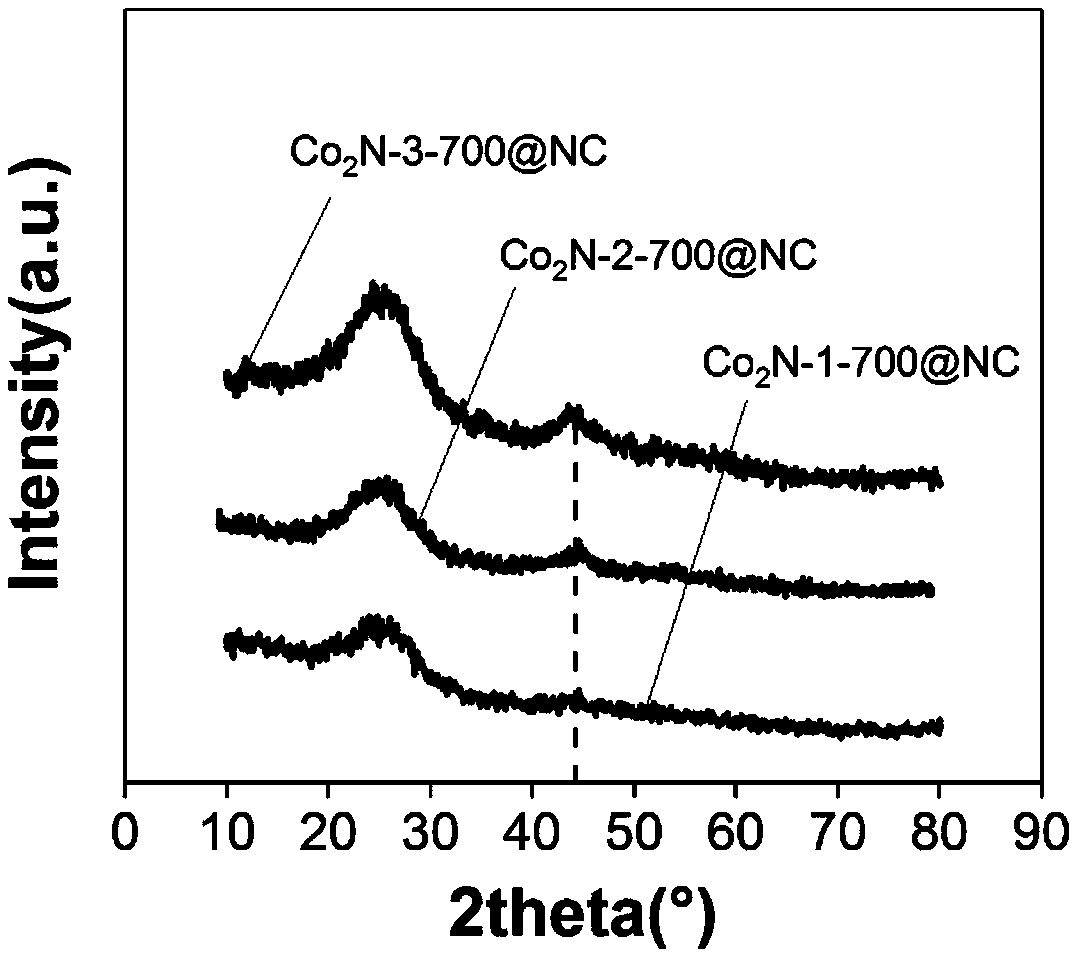
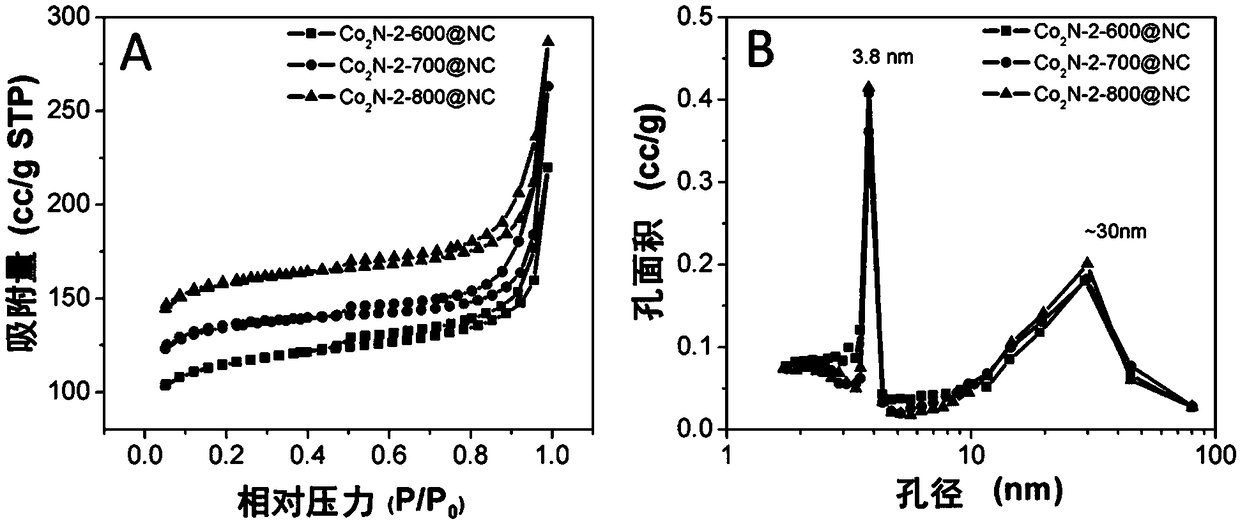
A cobalt nitride supported porous carbon catalyst for cathodic oxygen reduction reaction of a fuel cell and a preparation method thereof
A fuel cell cathode and catalyst technology, applied in battery electrodes, nanotechnology for materials and surface science, circuits, etc., can solve the problems of high cost of use, restricting the potential application prospects of fuel cells, etc., and achieve low cost and methanol resistance Strong poisoning ability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] (1) Dissolve 4.3mmol of aniline, 4.3mmol of pyrrole and 0.2mmol of Triton X-100 in 60mL of deionized water to obtain a uniform mixture, then add 2mmol of cobalt nitrate hexahydrate into the above mixture and stir to obtain a new mixture liquid;
[0029] (2) Add 20 mL of ammonium persulfate aqueous solution with a concentration of 0.05 g / mL pre-cooled at 3°C to the mixed solution obtained in step (1), and immediately place the mixed solution at 3°C for 24 hours to polymerize. After completion, filter, wash, and dry to obtain a black intermediate product;
[0030] (3) Put the black intermediate product obtained in step (2) in a tube furnace, then seal it and feed ammonia gas at 50mL / min, raise the temperature to 700°C at 5°C / min after 1 hour, and continue the high temperature treatment at 700°C After 3 hours, the temperature was lowered to room temperature, and the cobalt nitride-supported porous carbon catalyst was obtained, which was named as Co 2 N-2-700@NC.
Embodiment 2
[0032] (1) Dissolve 4.3mmol of aniline, 4.3mmol of pyrrole and 0.2mmol of Triton X-100 in 60mL of deionized water to obtain a uniform mixture, then add 1mmol of cobalt nitrate hexahydrate to the above mixture and stir to obtain a new mixture liquid;
[0033] (2) Add 20 mL of ammonium persulfate aqueous solution with a concentration of 0.05 g / mL pre-cooled at 3°C to the mixed solution obtained in step (1), and immediately place the mixed solution at 3°C for 24 hours to polymerize. After completion, filter, wash, and dry to obtain a black intermediate product;
[0034] (3) Put the black intermediate product obtained in step (2) in a tube furnace, then seal it and feed ammonia gas at 50mL / min, raise the temperature to 700°C at 5°C / min after 1 hour, and continue the high temperature treatment at 700°C After 3 hours, the temperature was lowered to room temperature, and the cobalt nitride-supported porous carbon catalyst was obtained, which was named as Co 2 N-1-700@NC.
Embodiment 3
[0036] (1) Dissolve 4.3mmol of aniline, 4.3mmol of pyrrole and 0.2mmol of Triton X-100 in 60mL of deionized water to obtain a uniform mixture, then add 3mmol of cobalt nitrate hexahydrate into the above mixture and stir to obtain a new mixture liquid;
[0037] (2) Add 20 mL of ammonium persulfate aqueous solution with a concentration of 0.05 g / mL pre-cooled at 3°C to the mixed solution obtained in step (1), and immediately place the mixed solution at 3°C for 24 hours to polymerize. After completion, filter, wash, and dry to obtain a black intermediate product;
[0038] (3) Put the black intermediate product obtained in step (2) in a tube furnace, then seal it and feed ammonia gas at 50mL / min, raise the temperature to 700°C at 5°C / min after 1 hour, and continue the high temperature treatment at 700°C After 3 hours, the temperature was lowered to room temperature, and the cobalt nitride-supported porous carbon catalyst was obtained, which was named as Co 2 N-3-700@NC.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com