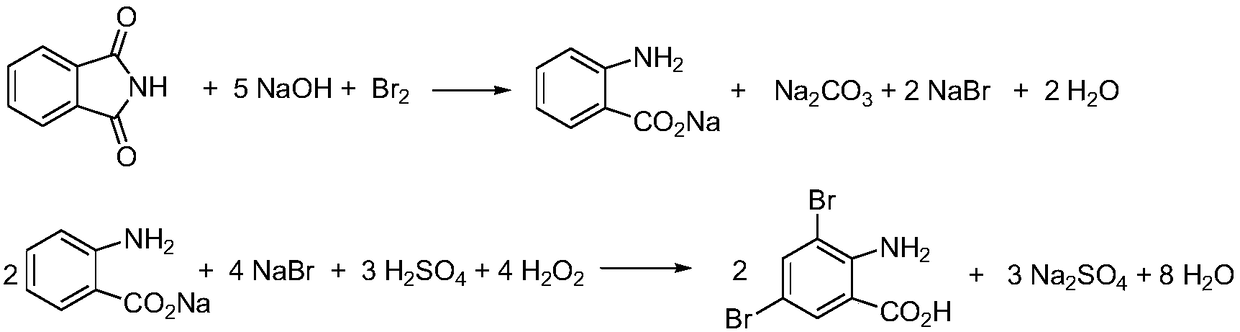
Preparation method for ambroxol hydrochloride intermediate 3,5-dibromo-2-aminobenzoic acid
A technology of aminobenzoic acid and ammonium bromide hydrochloride, which is applied in the field of preparation of ammonium bromide hydrochloride intermediate 3,5-dibromo-2-aminobenzoic acid, can solve the problems of large environmental pollution and high production cost, and achieve environmental protection Friendly, low production cost, low solubility effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] At room temperature, dissolve 6.0g (0.041mol) of phthalimide and 15.6g (0.39mol) of NaOH into 80g of water, cool down to -5-0°C, add 2.4mL (0.047mol) of liquid bromine dropwise, and Insulate and react at -5~0°C for 30 minutes. Raise the temperature of the reaction system to 70°C and keep it warm for 5-10 minutes, cool to room temperature, add 50mL (0.36mol) of 50% sulfuric acid dropwise, cool down to -5-0°C, add 9.6g (0.099mol) of 35% hydrogen peroxide dropwise, and The reaction was incubated at -5~0°C for 1 h, and then raised to room temperature to continue the reaction for 11 h. Filtration, the solid was washed with 2×30mL H 2 O was washed and dried to obtain 10.9 g of 3,5-dibromo-2-aminobenzoic acid light yellow solid with a yield of 90.8%.
Embodiment 2
[0035] At room temperature, dissolve 6.0g (0.041mol) of phthalimide and 22.2g (0.39mol) of KOH into 80g of water, cool down to -5-0°C, add 2.4mL (0.047mol) of liquid bromine dropwise, and Insulate and react at -5~0°C for 30 minutes. Raise the temperature of the reaction system to 70°C and keep it warm for 5-10 minutes, cool to room temperature, add 50mL (0.36mol) of 50% sulfuric acid dropwise, cool down to -5-0°C, add 9.6g (0.099mol) of 35% hydrogen peroxide dropwise, and Insulate and react at -5~0°C for 1 h, then rise to room temperature and continue to react for 12 h. After filtration, the solid was washed with 2×30 mL H2O, and dried to obtain 10.7 g of 3,5-dibromo-2-aminobenzoic acid as a pale yellow solid, with a yield of 89.2%.
Embodiment 3
[0037] At room temperature, dissolve 6.0g (0.041mol) of phthalimide and 15.6g (0.39mol) of NaOH into 80g of water, cool down to -5~0°C, add 2.2mL (0.043mol) of liquid bromine dropwise, and Insulate and react at -5~0°C for 30 minutes. Raise the temperature of the reaction system to 70°C and keep it warm for 5-10 minutes, cool to room temperature, add 50mL (0.36mol) of 50% sulfuric acid dropwise, cool down to -5-0°C, add 9.6g (0.099mol) of 35% hydrogen peroxide dropwise, and Insulate the reaction at -5~0°C for 1 h, then rise to room temperature and continue the reaction for 10 h. After filtration, the solid was washed with 2×30 mL H2O, and dried to obtain 10.2 g of 3,5-dibromo-2-aminobenzoic acid as a pale yellow solid, with a yield of 85.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com