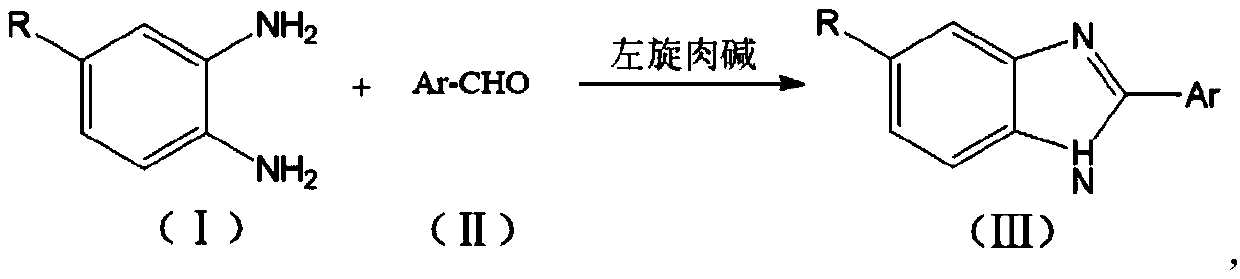
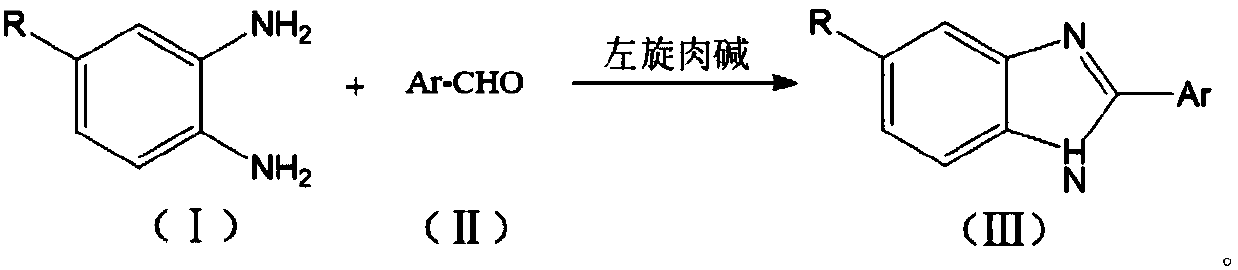
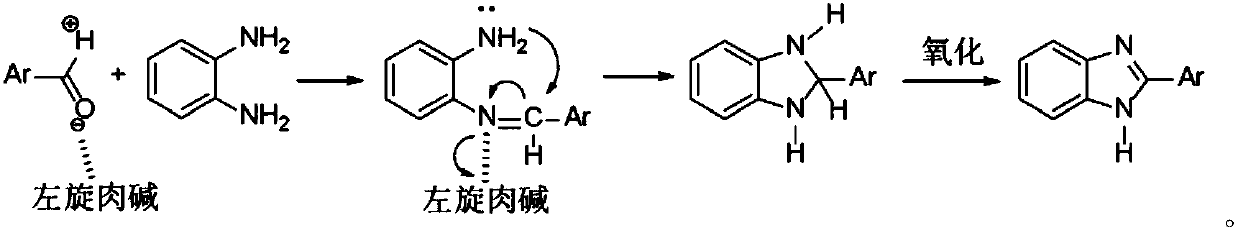
Synthetic method of benzimidazole compounds
A synthesis method and technology of benzimidazole, which are applied in the field of synthesis of benzimidazole compounds, can solve the problems of inability to recycle and reuse catalysts, complicated product post-processing, difficult catalyst preparation, etc., and achieve good practical value and social and economic efficiency, The effect of saving cost and labor input, and easy operation of post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of synthetic method of benzimidazole compound, its reaction formula is as follows:
[0033]
[0034] Include the following steps:
[0035] 1) Add o-phenylenediamine (108mg, 1.0mmol), p-chlorobenzaldehyde (141mg, 1.0mmol) and L-carnitine (16.1mg, 0.1mmol) into 5mL of 95% ethanol, heat at 60°C under electromagnetic stirring Reaction 2h, obtain crude product;
[0036] 2) The crude product obtained in step 1) was filtered, dried at 90° C. for 1 h, and recrystallized with ethanol to obtain a white solid with a yield of 97% and a purity of 99%.
[0037] The product is subjected to nuclear magnetic resonance detection and main element analysis, and the data are as follows:
[0038] 1 H-NMR (400MHz, DMSO-d 6 ): δ13.03(s,1H),8.23(d,2H,J=8.5Hz),7.70-7.63(m,3H),7.55(d,1H,J=7.2Hz),7.26-7.23(m, 2H);
[0039] 13 C NMR (100MHz, DMSO-d 6 ): δ149.8, 143.5, 134.5, 134.3, 129.0, 128.7, 128.1, 122.6, 121.8, 118.7, 111.3;
[0040] Anal.Calcd.for C 13 h 9 ClN 2 : C 68.2...
Embodiment 2
[0043] A kind of synthetic method of benzimidazole compound, its reaction formula is as follows:
[0044]
[0045] Include the following steps:
[0046] 1) Add 4-nitro-o-phenylenediamine (153mg, 1.0mmol), p-chlorobenzaldehyde (141mg, 1.0mmol) and L-carnitine (16.1mg, 0.1mmol) into 5mL of 95% ethanol, under electromagnetic stirring , heated at 60°C for 2h to obtain a crude product;
[0047] 2) The crude product obtained in step 1) was filtered, dried at 60° C. for 2 h, and recrystallized with ethanol to obtain a yellow solid with a yield of 98% and a purity of 99%.
[0048] The product is subjected to nuclear magnetic resonance detection and main element analysis, and the data are as follows:
[0049] 1 H-NMR (400MHz, DMSO-d 6 ): δ13.66(s, 1H), 8.81(s, 1H), 8.12(d, J=9.0Hz, 1H), 8.01(d, J=9.0Hz, 1H), 7.52(d, J=9.0Hz ,2H),7.43(d,J=9.0Hz,2H);
[0050] 13 C NMR (100MHz, DMSO-d 6 )δ158.4, 151.1, 135.9, 135.2, 133.3, 131.6, 129.9, 125.0, 124.4, 113.2, 112.8;
[0051] Ana...
Embodiment 3
[0054] A kind of synthetic method of benzimidazole compound, its reaction formula is as follows:
[0055]
[0056] Include the following steps:
[0057] 1) Add o-phenylenediamine (108mg, 1.0mmol), p-methoxybenzaldehyde (136mg, 1.0mmol) and L-carnitine (16.1mg, 0.1mmol) into 5mL of 95% ethanol, under electromagnetic stirring, 60 The reaction was heated at ℃ for 1 h to obtain the crude product;
[0058] 2) The crude product obtained in step 1) was filtered, dried at 70°C for 3 hours, and recrystallized with ethanol to obtain a white solid with a yield of 95% and a purity of 99%.
[0059] The product is subjected to nuclear magnetic resonance detection and main element analysis, and the data are as follows:
[0060] 1 H NMR (400MHz, DMSO-d 6 ): δ12.75(s, 1H), 8.09(d, 2H, J=8.7Hz), 7.55(m, 2H), 7.19-7.16(m, 2H), 7.10(d, 2H, J=8.8Hz) ,3.83(s,3H);
[0061] 13 C NMR (100MHz, DMSO-d 6 ): δ160.3, 151.0, 143.4, 134.7, 127.7, 122.3, 121.8, 121.4, 118.1, 114.0, 110.8, 55.1;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com