Process for producing alpha-halo ketones, alpha-halohydrine and epoxides
A halogenated ketone and a production method technology are applied in the field of preparing optically active α-halogenated ketones, α-halogenated alcohols and epoxides, and can solve the problem of not establishing an industrial production method for chlorinated ketones, difficult to implement on an industrial scale, unsatisfactory, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
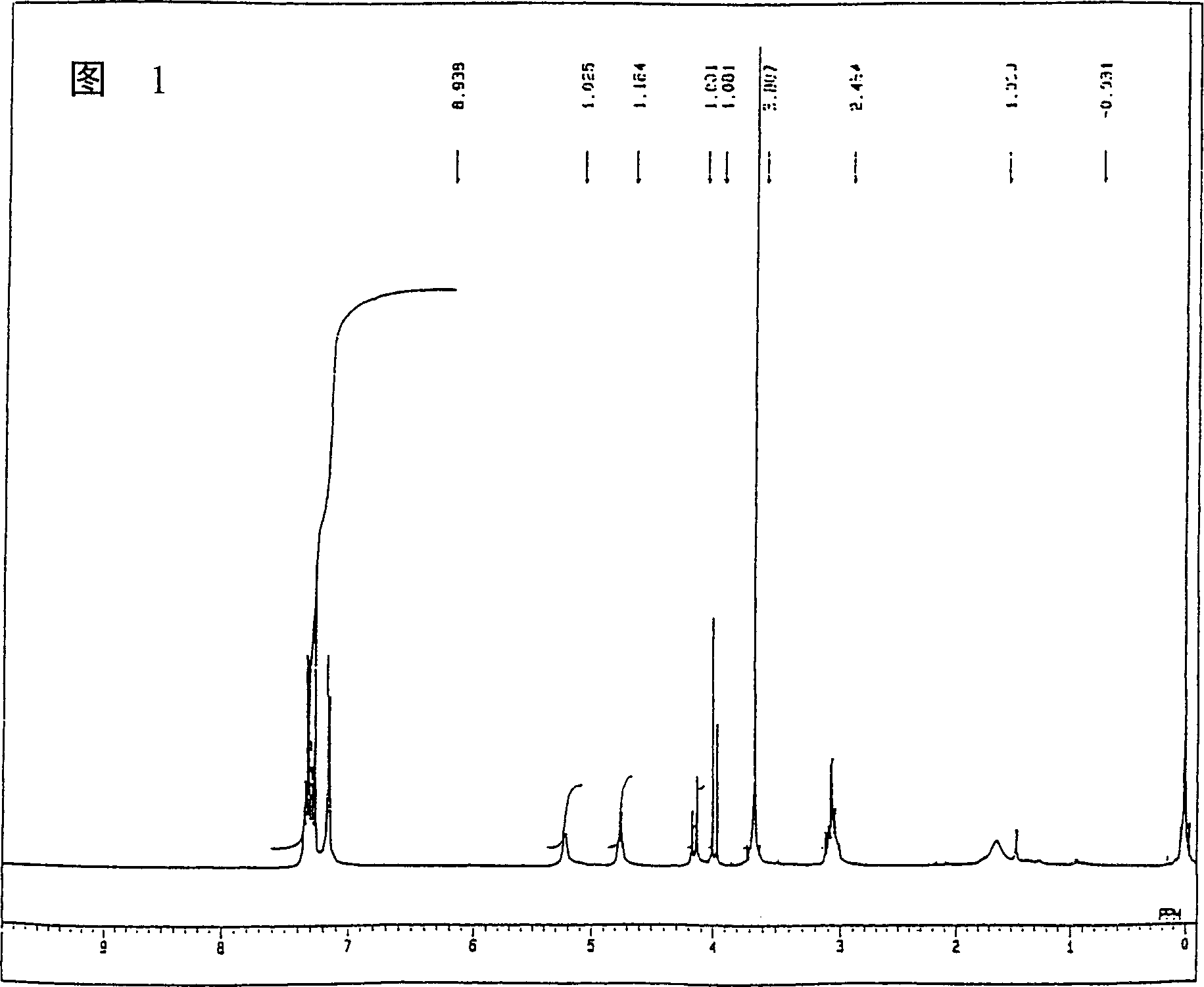
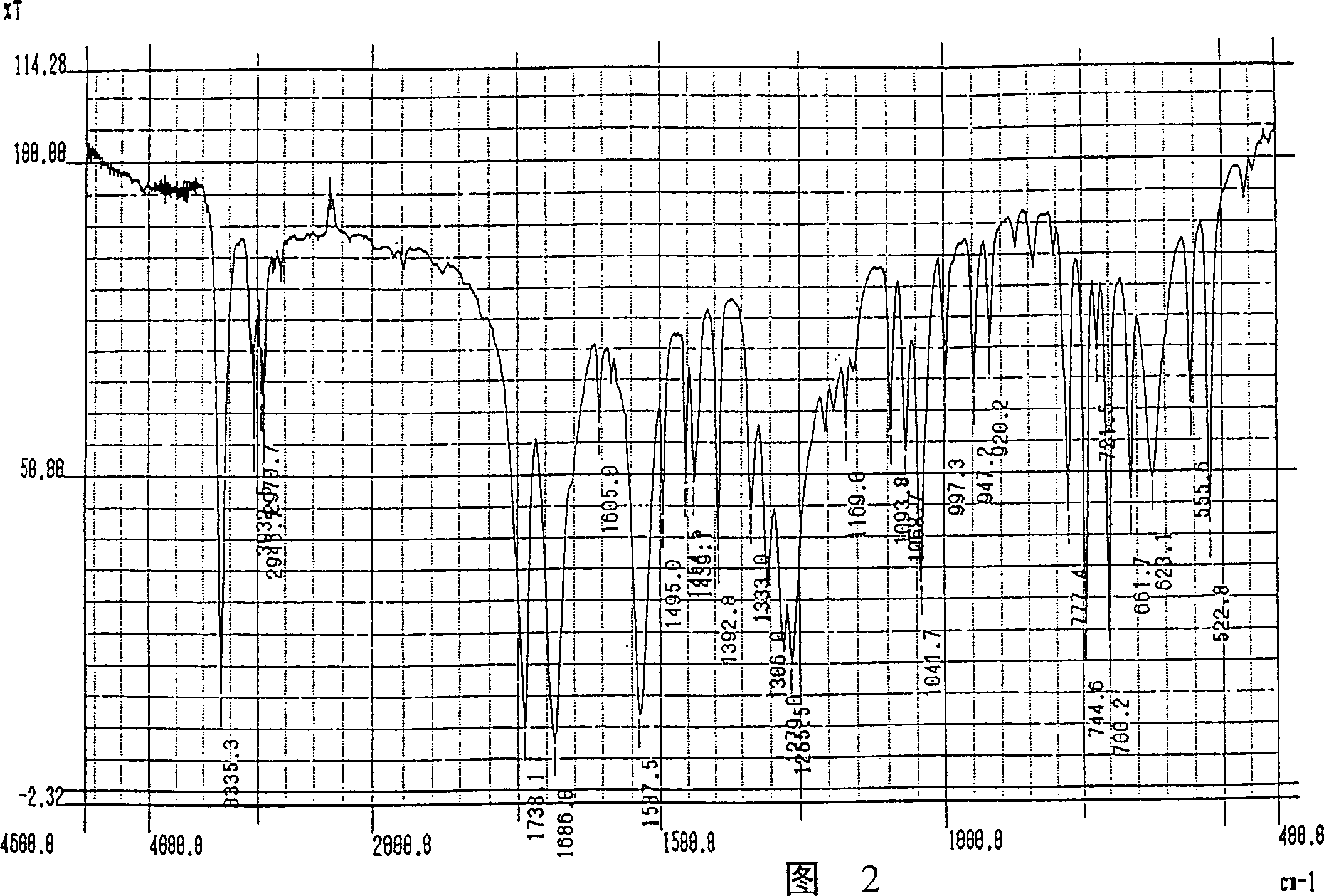
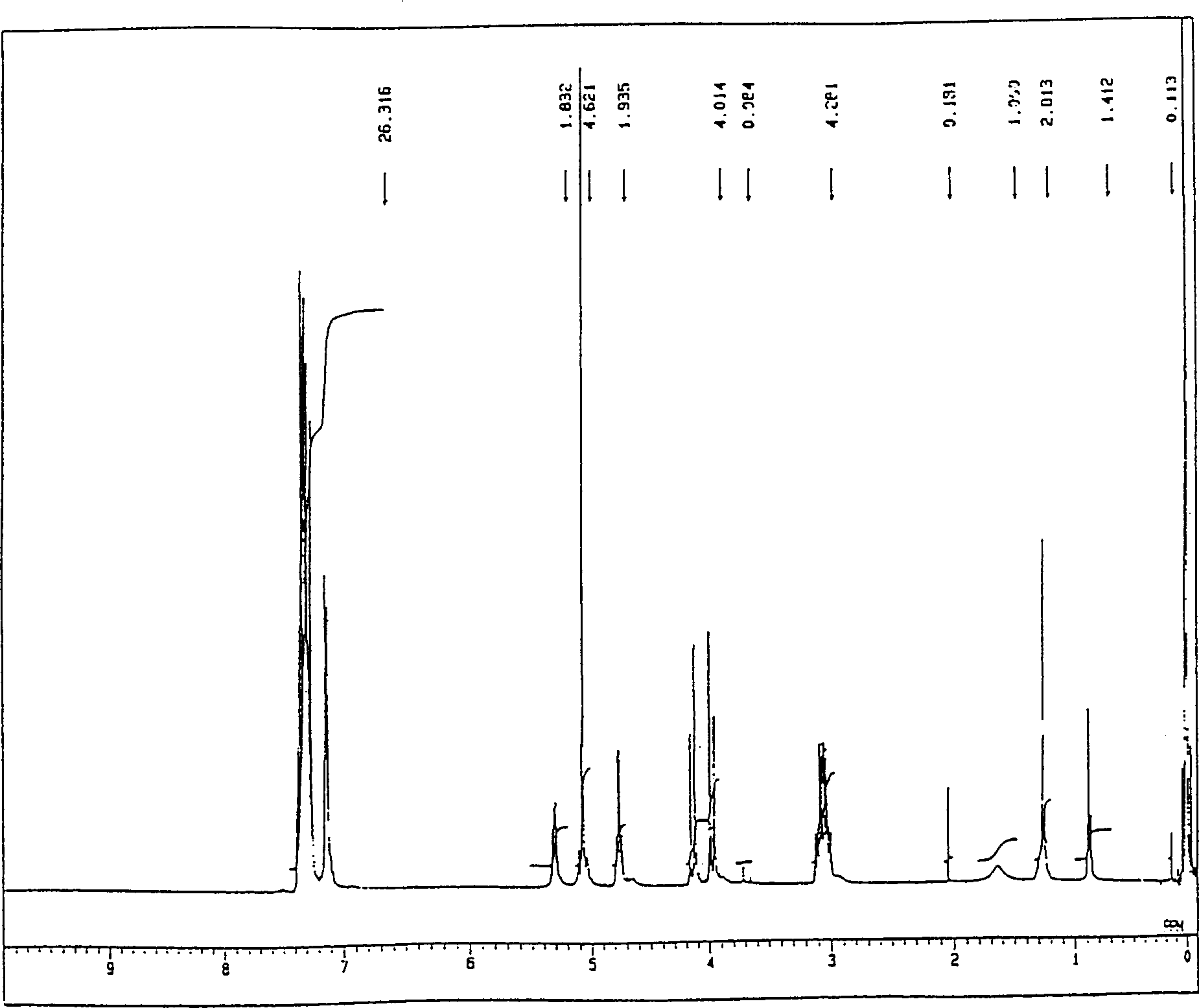
Image
Examples
Embodiment 1
[0088] The production of embodiment 1 phenacyl chloride (I)
[0089] Under a nitrogen atmosphere, diisopropylamine (2.42 g, 24.0 mmol) was dissolved in tetrahydrofuran (20 ml), and the solution was cooled to 0°C. To this solution was added n-butyllithium (1.6M / hexane, 13.8ml, 22.0mmol), and the mixture was stirred for 10 minutes. After cooling the solution to -30°C, monochloroacetic acid (0.946 g, 10.0 mmol) was added, and the mixture was stirred for 30 minutes. To the solution was added ethyl benzoate (0.514 g, 3.33 mmol), and the mixture was stirred at -30°C for 30 minutes. The temperature of the mixture was then raised to 25°C for 30 minutes. The reaction mixture was poured into 1N-HCl (50ml), extracted with ethyl acetate (50ml×2). The extracts were sequentially washed with saturated NaHCO 3 It was washed with aqueous solution (50ml×1) and water (50ml×1), and then dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated to give a pale y...
Embodiment 2-7
[0090] The production of embodiment 2-7 phenacyl chloride (I)
[0091] Examples 2-7 were carried out in substantially the same steps as in Example 1 with the following reaction temperatures and times.
[0092] Example
Embodiment 8
[0093] The production of embodiment 8 phenacyl chloride (I)
[0094] Diisopropylamine (0.668 g) was added to 6.67 ml of 0.9 M n-butylmagnesium chloride / tetrahydrofuran (6 mmol) under nitrogen atmosphere. The mixture was stirred at room temperature for 4 hours, then it was cooled to 0°C. A solution of 284 mg (3.0 mmol) of monochloroacetic acid and 136 mg (1 mmol) of methyl benzoate in tetrahydrofuran (5 ml) was added dropwise over 5 minutes. The mixture was stirred at 0°C for 30 minutes. The temperature was then allowed to rise to room temperature and stirred for an additional 30 minutes. The reaction mixture was poured into 1N-HCl (30ml), extracted with ethyl acetate (30ml×2). The extracts were sequentially washed with saturated NaHCO 3 It was washed with aqueous solution (30ml×1) and water (30ml×1), and then dried over anhydrous magnesium sulfate. After filtration, the filtrate was concentrated to give a pale yellow oil. HPLC analysis of the oil compared to an authentic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com