Human stem cell derived endothelial cells, endothelial- hepatocyte co-culture system and uses thereof
An endothelial cell, co-culture technique for use in cell biology and biochemistry that addresses issues such as expensive ethics, slow animal testing, and reduced effectiveness of therapeutic strategies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Example 1: Materials and methods
[0118] hPSC culture and maintenance
[0119] hPSCs were passaged using a mild cell dissociation agent (Stemcell Technologies, Cat. No. 07174) and seeded on Matrigel-coated plates in mTeSR1 medium (Stemcell Technologies, Cat. No. 05850). H9-ESCs and IMR90-iPSCs were purchased from WiCell Institute. BJ-iPSCs were kindly provided by collaborators' laboratories.
[0120] Generation of endothelial cells from hPSCs
[0121] After hPSC colonies attach, they are first induced to drive early mesoderm differentiation for 36 hours using a chemically defined medium supplemented with recombinant human FGF2 (20 ng / ml, R&D Systems (R&D Systems, Cat. No. 233-FB), LY294002 (10 μΜ, Sigma, Cat. No. L9908), and human recombinant BMP4 (10 ng / ml, R&D Systems, Cat. No. 314-BP). Lateral plate mesoderm was further induced for 3.5 days in medium supplemented with human recombinant FGF2 (20 ng / ml) and BMP4 (50 ng / ml), with medium changes every 2 days. O...
Embodiment 2
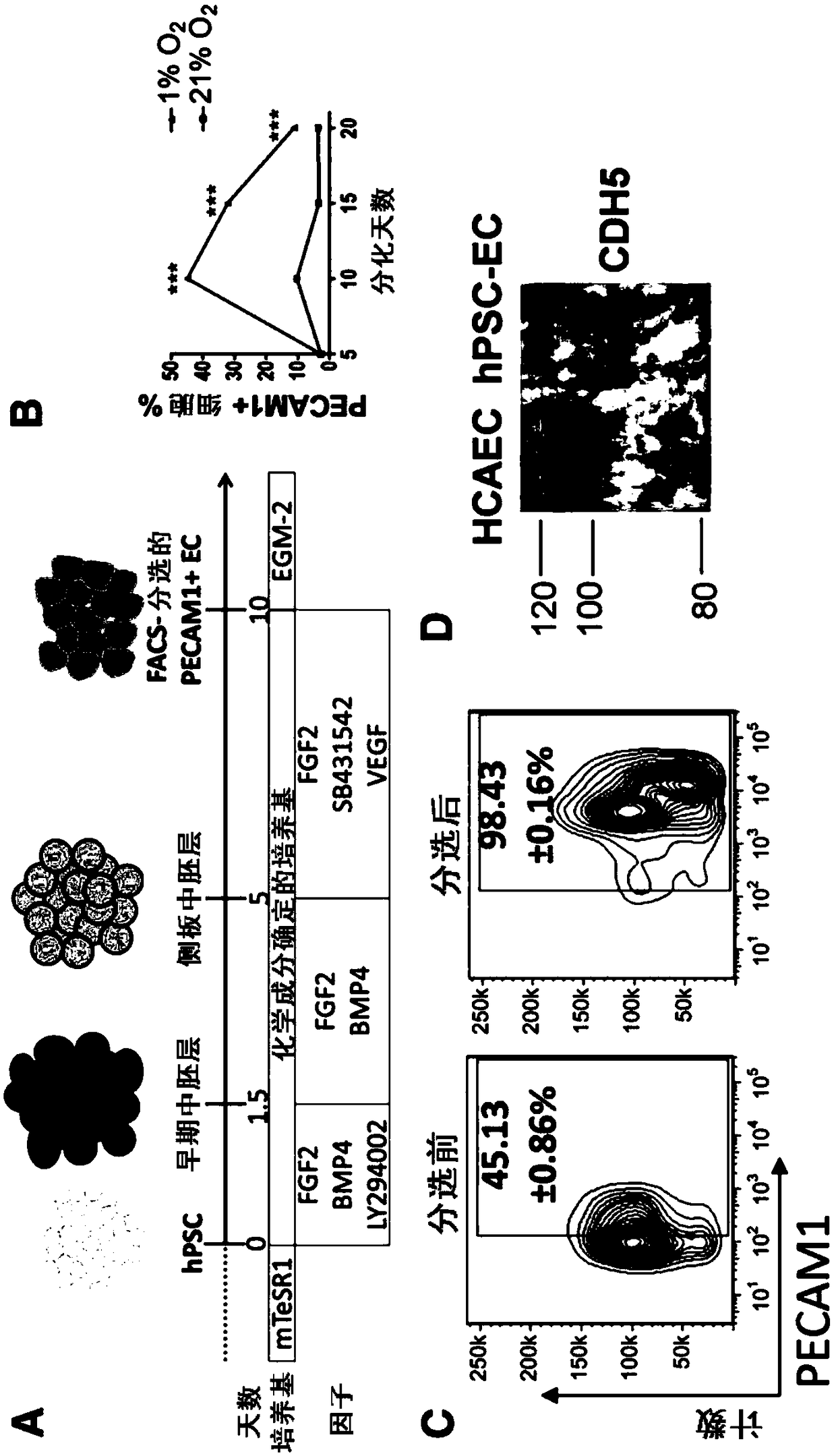
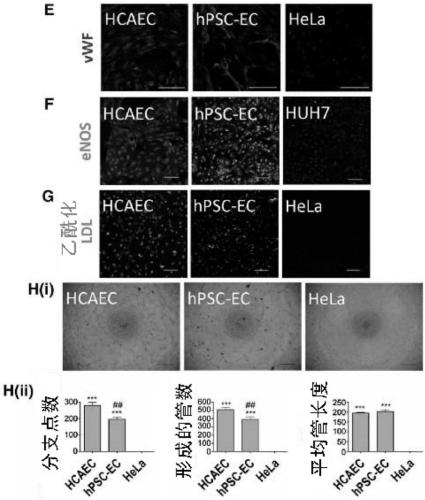
[0153] Example 2: Derivation of functional endothelial cells from human pluripotent stem cells
[0154] Lateral plate mesoderm is the precursor tissue of the vascular lineage. Lateral plate mesoderm was induced with fibroblast growth factor 2 (FGF2), bone morphogenetic protein 4 (BMP4), and a phosphatidylinositol 3-kinase inhibitor (LY294002) for 5 days ( figure 1 A). A combination of factors is used to drive endothelial specification. Inhibition of transforming growth factor beta (TGF-β) using the small molecule SB431542 enhanced endothelial differentiation of hPSCs, possibly by counteracting the growth of parietal cells that might arise from common cardiovascular progenitors. FGF2 and vascular endothelial growth factor (VEGF) are mitogens for promoting angiogenesis and endothelial development. Hypoxic conditions increase the efficiency of endothelial differentiation because upregulation of hypoxia-inducible factors triggers downstream targets that are important in early v...
Embodiment 3
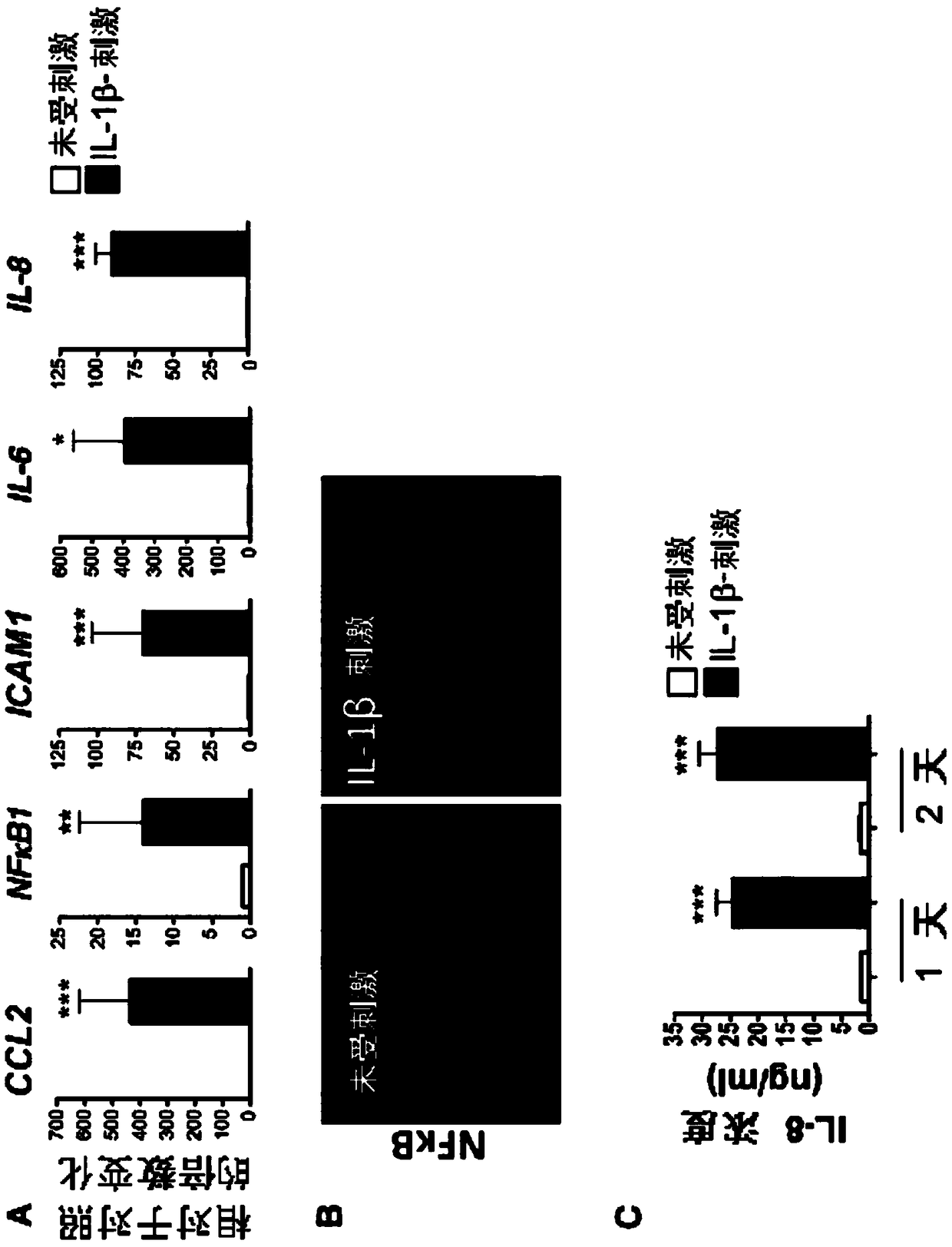
[0156] Example 3: HPSC-derived endothelial cells respond to inflammatory stimuli
[0157] Inflammation is a hallmark of atherosclerosis. To recapitulate atherosclerosis-associated phenotypes in hPSC-ECs, the inflammatory cytokine interleukin-1β (IL-1β), which is widely implicated in atherosclerosis, was used. After stimulation with recombinant human IL-1β, hPSC-ECs responded in which inflammatory genes were significantly upregulated ( figure 2 A). Nuclear translocation of nuclear factor kappa B (NFκB), which activates a major pro-inflammatory mediator, has been observed in human atherosclerotic lesions. Likewise, nuclear translocation of NFκB was evident in hPSC-ECs following stimulation with IL-1β ( figure 2 B). Interleukin 8 (IL-8) production in the conditioned medium of IL-1β-stimulated hPSC-ECs was significantly higher than that of unstimulated cells ( figure 2 C). In addition to H9-EC, the inventors of the present application also verified that BJ-EC and IMR90-EC...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com