Taxane derivative TM-2 micellar injection, preparation method thereof and application thereof
A technology of TM-2 and taxanes, applied in the field of medicine, can solve the problems of complex preparation process of lipid microsphere injection, unstable preparation, high drug concentration, etc., so as to avoid severe allergic reactions and peripheral neuropathy, Good stability and high drug loading effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
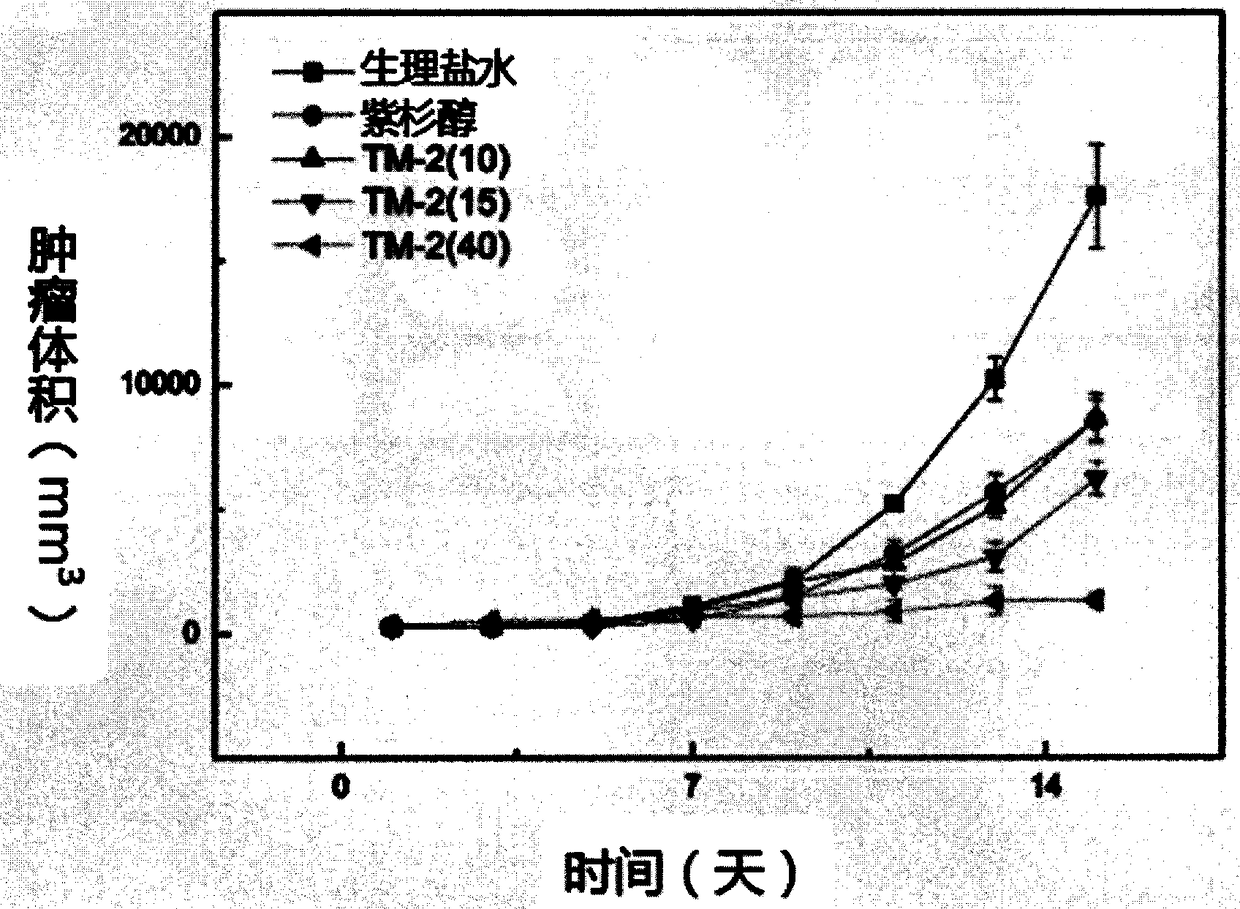
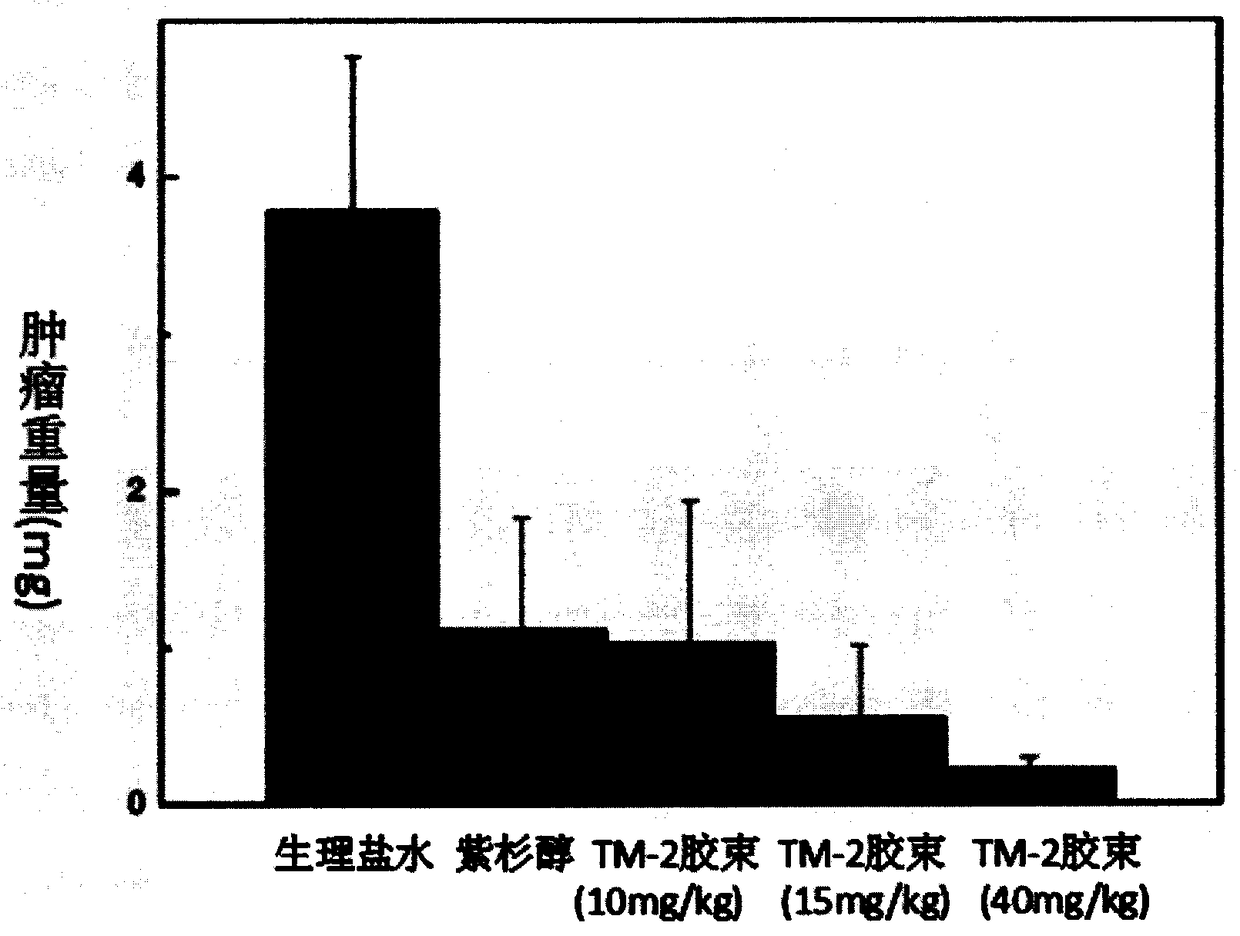
Examples
Embodiment 1
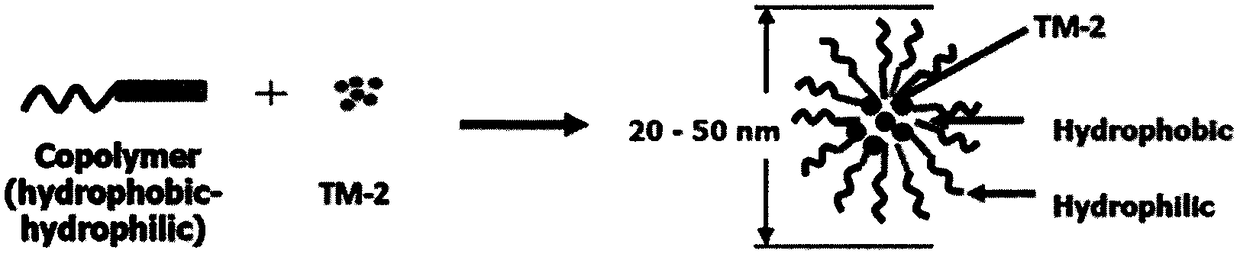
[0038] This embodiment provides a taxane derivative TM-2 micellar injection, which includes the following components: mPEG-PLA, TM-2, lyoprotectant, water for injection; wherein mPEG-PLA, TM- 2. The composition of the freeze-drying protective agent according to the mass percentage is: mPEG-PLA 10.8%, TM-2 2.7%, and the freeze-drying protective agent 86.5%. The mPEG-PLA is a block copolymer of polyethylene glycol monomethyl ether and polylactic acid, wherein the molar ratio between the polyethylene glycol monomethyl ether and the polylactic acid is 1:1; The molecular weight of glycol monomethyl ether is 2000, the molecular weight of the polylactic acid is 2000, and the molecular weight of the mPEG-PLA is 4000.
[0039] The lyoprotectant is used in combination with PEG4000 and sucrose, and the weight ratio of the two is 1:1.
[0040] The preparation method of the taxane derivative TM-2 micellar injection comprises the following steps:
[0041] (1) According to the above-mentione...
Embodiment 2
[0047] This embodiment provides a taxane derivative TM-2 micellar injection, which includes the following components: mPEG-PLA, TM-2, lyoprotectant, water for injection; wherein mPEG-PLA, TM- 2. The composition of the freeze-drying protective agent according to the mass percentage is: mPEG-PLA 15.1%, TM-2 3.1%, and the freeze-drying protective agent 81.8%. The mPEG-PLA is a block copolymer of polyethylene glycol monomethyl ether and polylactic acid, wherein the molar ratio between the polyethylene glycol monomethyl ether and the polylactic acid is 1:1; The molecular weight of glycol monomethyl ether is 4000, the molecular weight of the polylactic acid is 5000, and the molecular weight of the mPEG-PLA is 9000.
[0048] The freeze-drying protectant is a combination of poloxamer 188, sucrose and hydroxyethyl starch, and the weight ratio of the three is 1:1:1.
[0049] The preparation method of the taxane derivative TM-2 micellar injection comprises the following steps:
[0050]...
Embodiment 3
[0056] This embodiment provides a taxane derivative TM-2 micellar injection, which includes the following components: mPEG-PLA, TM-2, lyoprotectant, water for injection; wherein mPEG-PLA, TM- 2. The composition of the freeze-drying protective agent according to the mass percentage is: mPEG-PLA 50%, TM-2 10.5%, and the freeze-drying protective agent 39.5%. The mPEG-PLA is a block copolymer of polyethylene glycol monomethyl ether and polylactic acid, wherein the molar ratio of the polyethylene glycol monomethyl ether to the polylactic acid is 1:1.25; The molecular weight of glycol monomethyl ether is 2000, the molecular weight of the polylactic acid is 2500, and the molecular weight of the mPEG-PLA is 4500.
[0057] The lyoprotectant is HAS.
[0058] The preparation method of the taxane derivative TM-2 micellar injection comprises the following steps:
[0059] (1) According to the above-mentioned mass percentage ingredients, first mix mPEG-PLA and TM-2 with dichloromethane to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com