Application of lutein and derivative thereof in preparing anti-glioma drug
A glioma and lutein technology, applied in the direction of anti-tumor drugs, drug combinations, active ingredients of hydroxyl compounds, etc., can solve the problems of unreported lutein anti-glioma activity and difficulties in artificial chemical synthesis of lutein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Extraction of Chemical Components in Porphyra laver and Screening of Active Parts
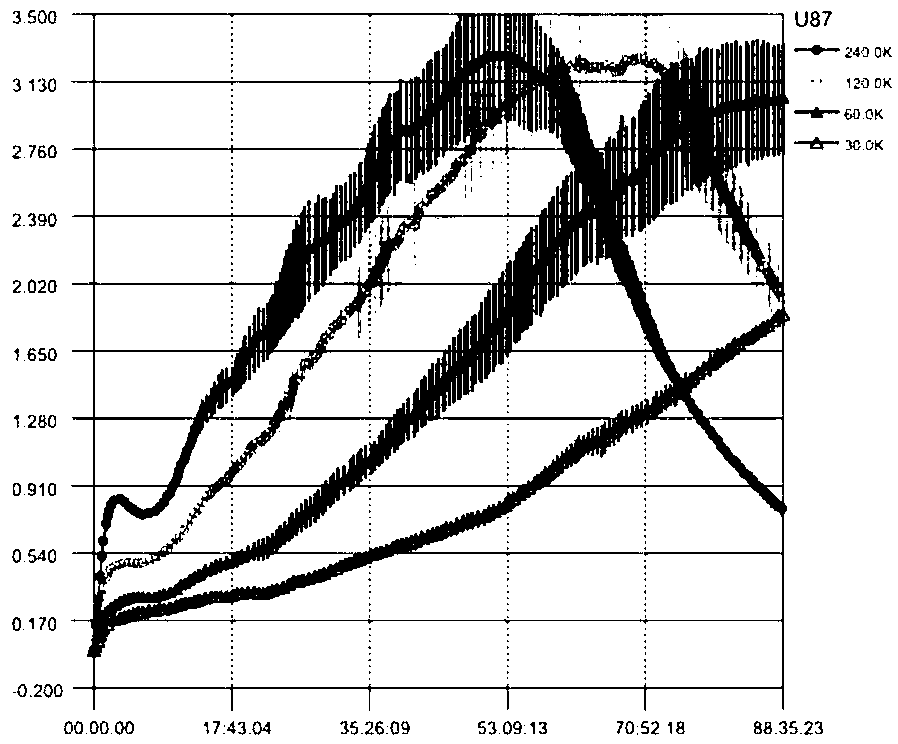
[0036] Grind Porphyra laver into powder, soak in 95% ethanol solution, the ratio of solid to liquid is 1:7, ultrasonic treatment for 30min, continuous leaching for 7 days, suction filtration of the leaching solution, concentration by rotary evaporation at 50°C under low pressure, Sequentially adopt an equal volume of petroleum ether, ethyl acetate and n-butanol to carry out static extraction, each organic phase is continuously extracted 3 times, each extraction is 4h, and each organic phase is combined respectively, and the concentrated solid extract by rotary evaporation under low pressure ( figure 1 ), the three extract phases were detected by thin layer chromatography ( figure 2 ). The proliferation curve of U87 cells was determined by RTCA method ( image 3 ), and finally determined that the optimal inoculation density of cells was 60,000 / mL, the drug addition time was 2...
Embodiment 2
[0039] Example 2 Separation, purification and structural identification of lutein
[0040] Preliminary separation and purification of the combined phase of petroleum ether and ethyl acetate extraction phases was carried out by silica gel column chromatography, and the steps were as follows: ① Sample mixing (sand making): Dissolve an appropriate amount of extract in a 500mL round-bottomed flask with appropriate amount of petroleum ether and ethyl acetate , add an appropriate amount of 200-300 mesh silica gel, and spin-steam to make sand. It is required to be completely adsorbed, loose and not agglomerated. ②Column packing: The dry method is used to load the sample, the height ratio of the sample sand to the silica gel is about 1:20, and the total height of the packed column is about 3 / 4 of the column height. Run the column with petroleum ether and compact it with a pressurizer. ③Elution: Gradient elution with petroleum ether-ethyl acetate eluent, the ratio is petroleum ether:e...
Embodiment 3
[0041] Embodiment 3 Lutein dehydration
[0042] The substrate lutein (1g, 1.76mmol) was added to a mixed solvent of 6ml concentrated hydrochloric acid and 60m water, and heated to reflux after the addition was complete. After the reaction was completed, it was extracted with diethyl ether (30ml×3), and the organic phases were combined, dried and then column chromatographed to obtain 0.8g of the target product with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com