A medicine for treating bone marrow hematopoietic system injury and repairing
A technology of medicine and bone marrow transplantation, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
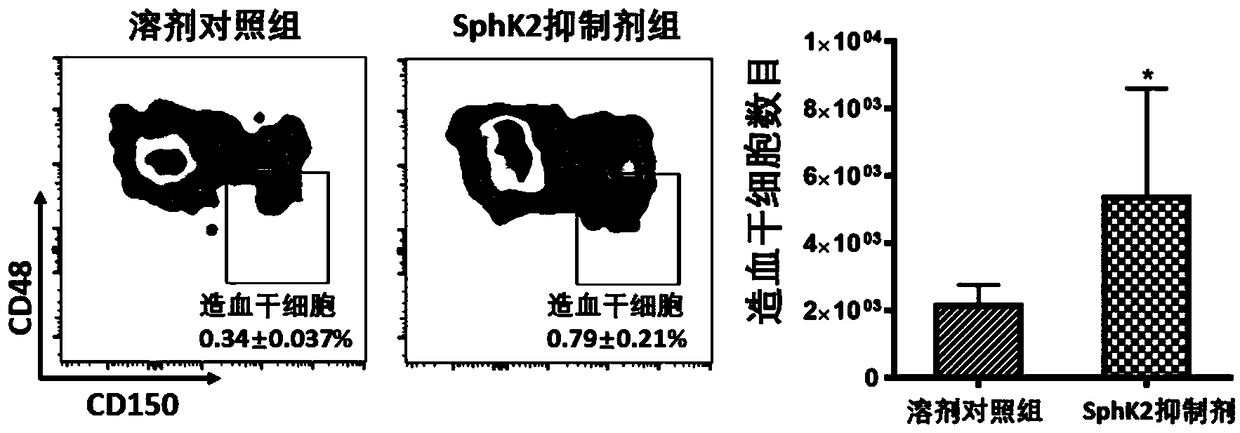
[0016] After bone marrow transplantation of hematopoietic stem cells, injection of SphK2 gene inhibitor ABC294640 significantly increased the number of hematopoietic stem cells.
[0017] Twenty-five 8-week-old C57 mice (purchased from the Experimental Animal Center of Sun Yat-Sen University) were selected as experimental mice and raised in a specific pathogen-free (SPF) environment. Take 5 of the C57 mice, kill them by neck dislocation, take their femurs and tibias, use a 1ml syringe to flush out the bone marrow in the bone marrow cavity, blow it into a single cell suspension, and lyse the red blood cell lysate for 2 minutes to remove the red blood cells, filter them with 70um Filter through a mesh to obtain a bone marrow single cell suspension. Another 20 C57 mice were subjected to X-ray radiation injury with a dose of 900cGy, and then each irradiated mouse was injected with 400,000 bone marrow single cell suspensions through the tail vein. Next, the mice that had undergone ...
Embodiment 2
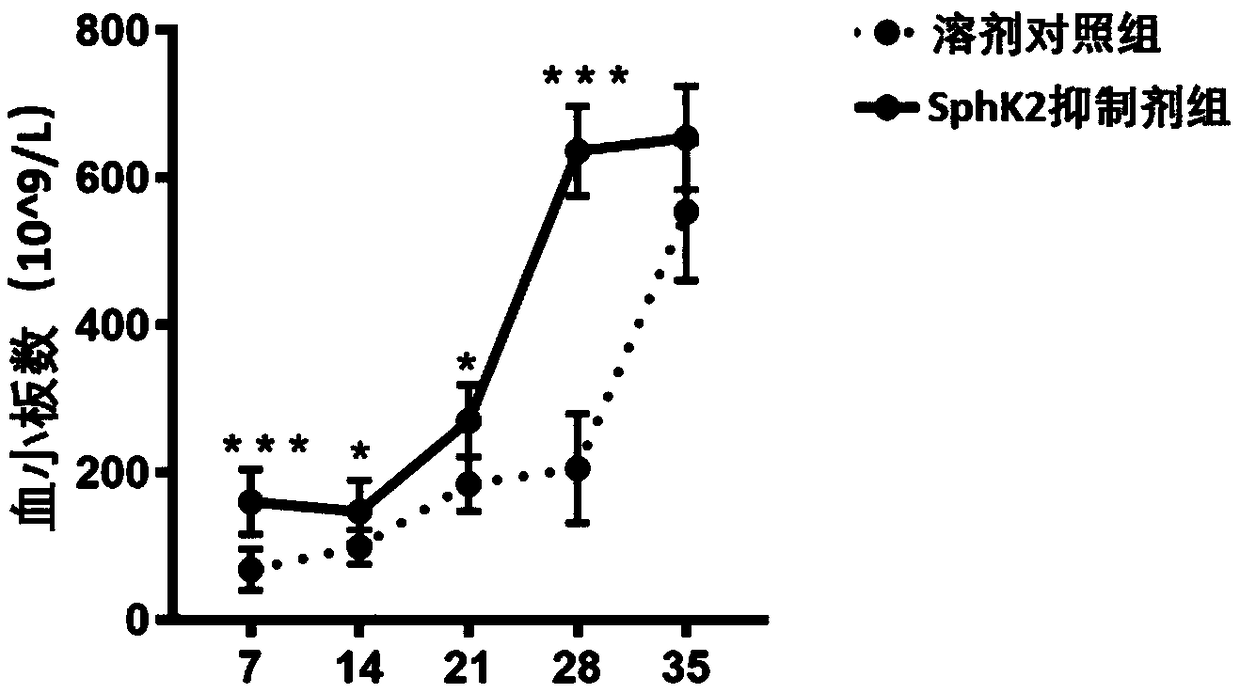
[0021] Inhibitor ABC294640 injection significantly accelerated platelet recovery after hematopoietic stem cell bone marrow transplantation.
[0022] The preparation of the SphK2 inhibitor was consistent with that in Example 1. Twenty 8-week-old C57 mice purchased from the Animal Center of Sun Yat-sen University were selected and divided into two groups, each with 10 mice. Same as in Example 1, the injection of the inhibitor ABC294640 and the solvent control was the same as in Example 1. The injection was also started on the second day after irradiation, and then every two days at a dose of 10 mg / kg until the end of the experiment. In the first week, the second week, the third week, the fourth week and the fifth week (the 7th, 14th, 21st, 28th, and 35th days), blood was taken from the tail vein of the mice in an anticoagulant tube, and blood routine detection was used Peripheral blood platelet count. We used Graphpad6.0 for t-test analysis, and marked the significance of the d...
Embodiment 3
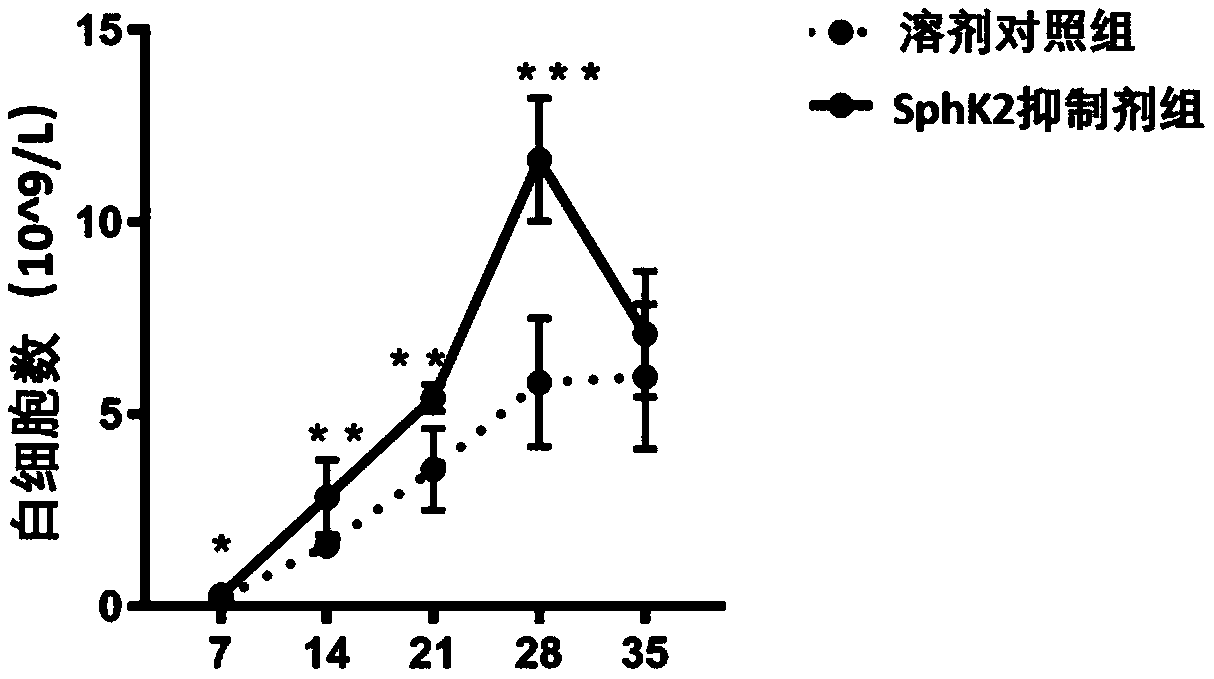
[0025] Inhibitor ABC294640 injection significantly accelerated the recovery of white blood cells after hematopoietic stem cell bone marrow transplantation.
[0026] The preparation of the SphK2 inhibitor was consistent with that in Example 1. Twenty 8-week-old C57 mice purchased from the Animal Center of Sun Yat-sen University were selected and divided into two groups, each with 10 mice. Same as in Example 1, the injection of the inhibitor ABC294640 and the solvent control was the same as in Example 1. The injection was also started on the second day after irradiation, and then every two days at a dose of 10 mg / kg until the end of the experiment. In the first week, the second week, the third week, the fourth week and the fifth week (the 7th, 14th, 21st, 28th, and 35th days), blood was taken from the tail vein of the mice in an anticoagulant tube, and blood routine detection was used Peripheral blood leukocyte count. We used Graphpad6.0 for t-test analysis, and marked the sign...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com