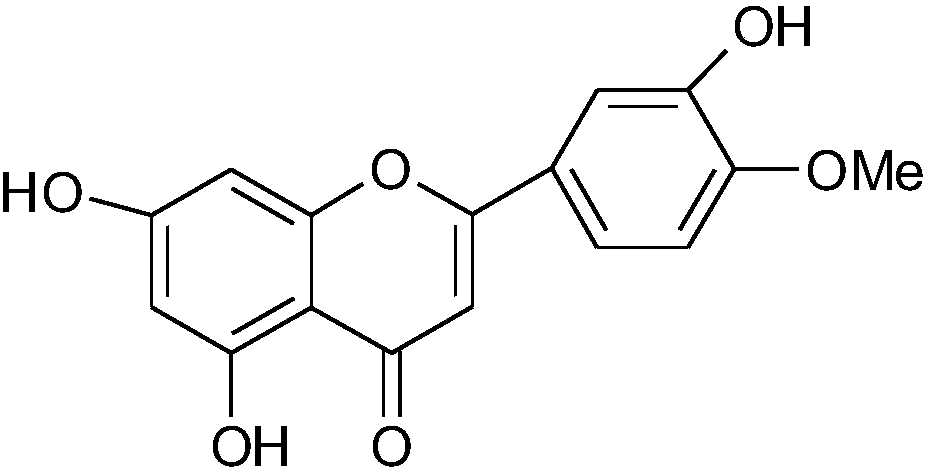
Semi-synthesis method of diosmetin
A geranyl lignin and semi-synthetic technology, applied in the direction of organic chemistry and the like, can solve the problems of low synthesis efficiency and high production cost, and achieve the effects of low production cost, high preparation purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
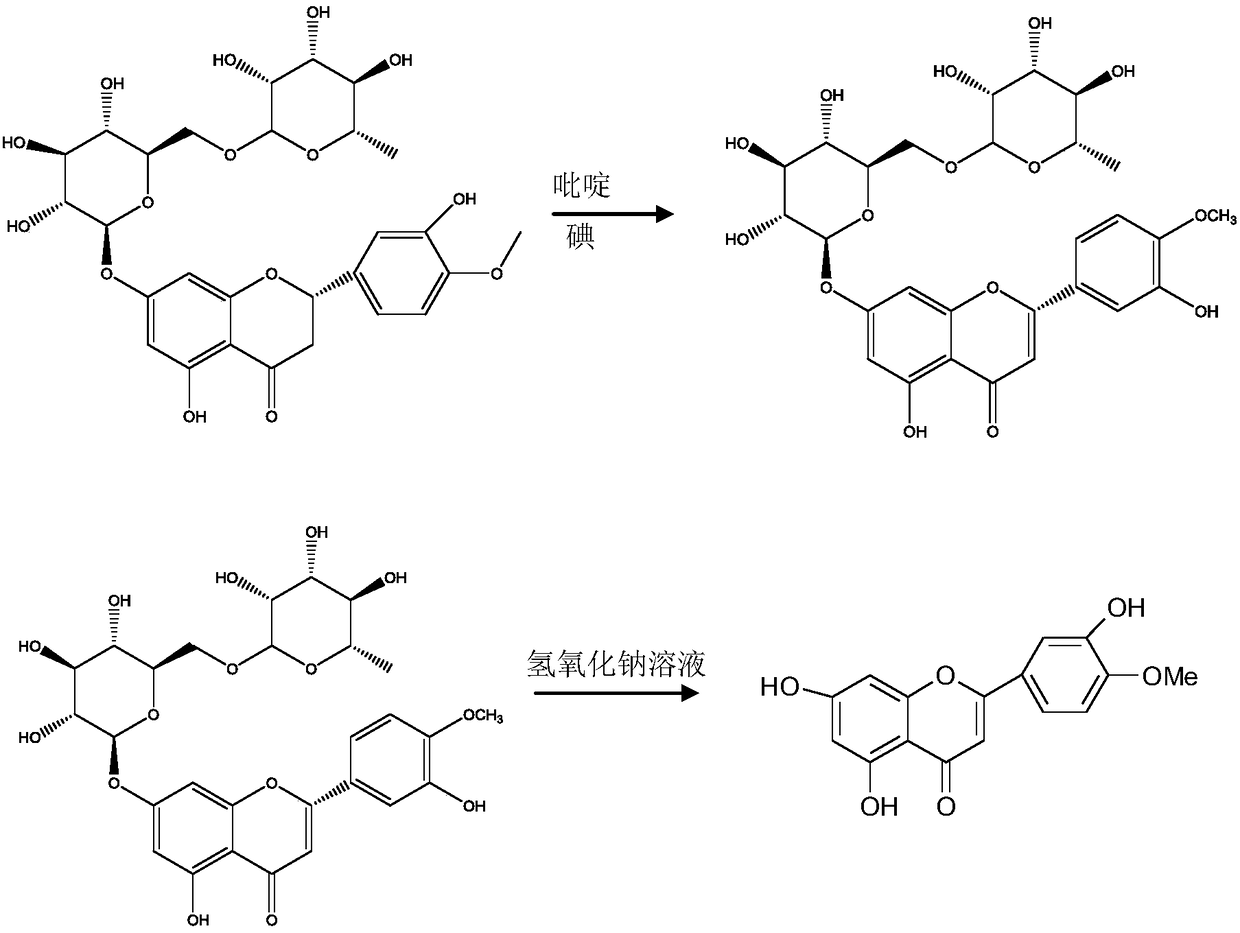
[0028] A semi-synthetic method for diosmin, the steps are as follows:
[0029] Step 1) Preparation of Neodiosmin
[0030] Put 300 kilograms of neohesperidin with a liquid phase content of 95%, 1800 kilograms of pyridine, and 240 kilograms of iodine into a 3000-liter reactor, heat up to 100 ° C for 12 hours, and monitor the neohesperidin content to below 2%. Recover pyridine under reduced pressure until no liquid is evaporated to obtain neodiosmin;
[0031] Step 2) prepare the crude product of diosmin
[0032] Add 1500 kilograms of sodium hydroxide solution with a mass concentration of 32% to the neodiosmin obtained in step 1), continue to heat up to 100° C. for 40 minutes of hydrolysis reaction, and monitor the content of neodiosmin to below 2% in the liquid phase. Add hydrochloric acid to the reaction solution to neutralize the pH value to 6-7, then cool down to 15°C-25°C to cool, centrifuge, collect the precipitate, and wash the precipitate with water to obtain 120 kg of c...
Embodiment 2
[0036] A semi-synthetic method for diosmin, the steps are as follows:
[0037] Step 1) Preparation of Neodiosmin
[0038] Put 100 kilograms of neohesperidin with a liquid phase content of 95%, 500 kilograms of pyridine, and 100 kilograms of iodine into a 1000-liter reactor, heat up to 105°C and react for 10 hours, and monitor the neohesperidin content below 2% in the liquid phase. Recover pyridine under reduced pressure until no liquid is evaporated to obtain neodiosmin;
[0039] Step 2) prepare the crude product of diosmin
[0040] Add 600 kg of sodium hydroxide solution with a mass concentration of 32% to the neodiosmin obtained in step 1), continue to heat up to 95°C for hydrolysis reaction for 50 minutes, monitor the content of neodiosmin below 2% in liquid phase, and add to Add hydrochloric acid to the reaction solution to neutralize it so that the pH value is 6-7, then cool down to 15°C-25°C, cool down, centrifuge, collect the precipitate, and wash the precipitate with...
Embodiment 3
[0044] A semi-synthetic method for diosmin, the steps are as follows:
[0045] Step 1) Preparation of Neodiosmin
[0046] Put 500 kilograms of neohesperidin with a liquid phase content of 95%, 3500 kilograms of pyridine, and 410 kilograms of iodine into a 5000-liter reactor, heat up to 110 ° C for 11 hours, and monitor the neohesperidin content to below 2%. Recover pyridine under reduced pressure until no liquid is evaporated to obtain neodiosmin;
[0047] Step 2) prepare the crude product of diosmin
[0048] Add 3000 kg of sodium hydroxide solution with a mass concentration of 32% to the neodiosmin obtained in step 1), continue to heat up to 100 ° C for 30 minutes of hydrolysis reaction, and monitor the neodiosmin content below 2% in the liquid phase. Add hydrochloric acid to the reaction solution to neutralize the pH value to 6-7, then cool down to 15°C-25°C to cool, centrifuge, collect the precipitate, and wash the precipitate with water to obtain 200 kg of crude geranoli...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com