A combined medicine capable of improving the therapeutic effect of polypeptide vaccine on HPV infected tumor and application thereof
A peptide vaccine and tumor treatment technology, applied in the field of biomedicine, can solve the problem of not improving the survival time of mice, and achieve the effect of extensive immunogenicity and easy production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0052] The preparation method of F1, F3 polypeptide cream of the present invention is as follows: Poloxamer 407 (molecular weight 12600, pH 6-7.4, batch number WPAK592B, BASF, Germany), poloxamer 188 (molecular weight 8400, pH 6-7, batch number WPAK539B, BASF, Germany) ;
[0053] Blank gel preparation: Add 46 grams of poloxamer 407 and 10 grams of poloxamer 188 into 200ml of distilled water, place at 4 degrees Celsius until poloxamer 407 and 188 are completely dissolved; stir well until the gel is formed. Add 10mg of F1 or F3 to 10ml blank gel. After complete dissolution, filter with a 0.22um filter.
[0054] The four polypeptides in the polypeptide composition of the present invention (hereinafter referred to as EX) are artificially synthesized by a biotechnology company using the Fmoc-Glu solid-phase method, and the crude peptides are purified by high performance liquid chromatography with a purity of >95% and freeze-dried at -20 ℃ and save for future use.
Embodiment 1
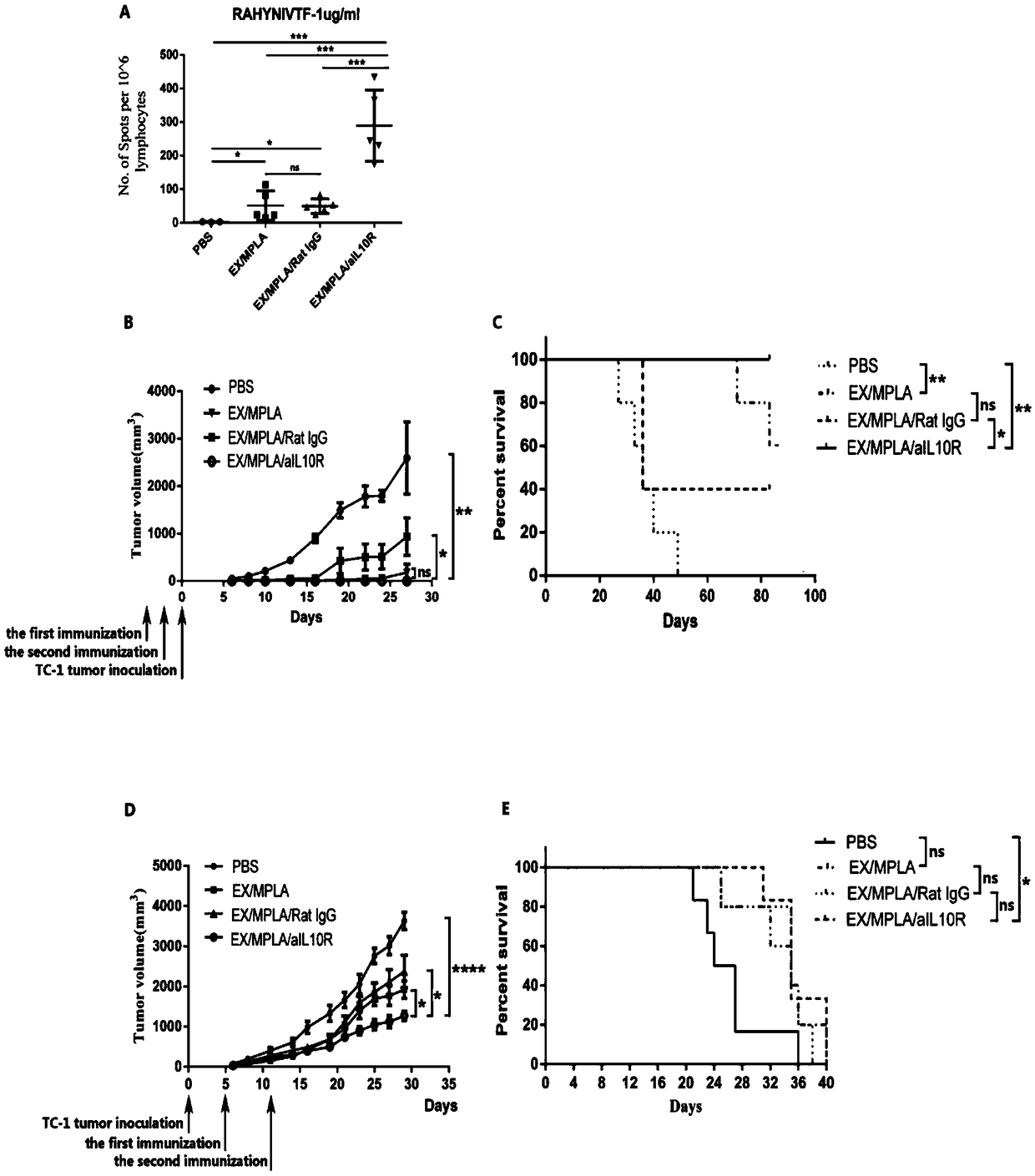
[0055] Example 1: Polypeptide composition combined with different Toll-like receptor ligands, or combined with aIL10R immunization
[0056] Experimental procedure
[0057] C57BL / 6 mice were randomly divided into 4 groups, group 1 was EX+MPLA; group 2 was EX+MPLA+Rat IgG; group 3 was EX+MPLA+aIL10R; group 4 was PBS control group.
[0058] The four groups of mice were subcutaneously immunized on the left abdomen, once a week, and immunized twice in total. The mice were sacrificed on the 6th day after the last immunization, the spleens of the mice were removed, and the antigen-specific CD8+ T cell response induced by the vaccine was detected by ELISPOT method.
[0059] the result shows:
[0060] 1. The 4 E7 polypeptide / MPLA / anti-interleukin 10 receptor antibody vaccine containing the full length of HPVE7 induces more antigen-specific CD8+ T cells than the vaccine without the interleukin 10 receptor antibody vaccine. See results figure 1 As shown in A.
[0061] 2. C57BL / 6 mic...
Embodiment 2
[0063] Example 2 F1 and F3 compositions can increase the effect of polypeptide vaccines with specific immune function in inhibiting the growth of TC-1 tumors
[0064] Experimental procedure
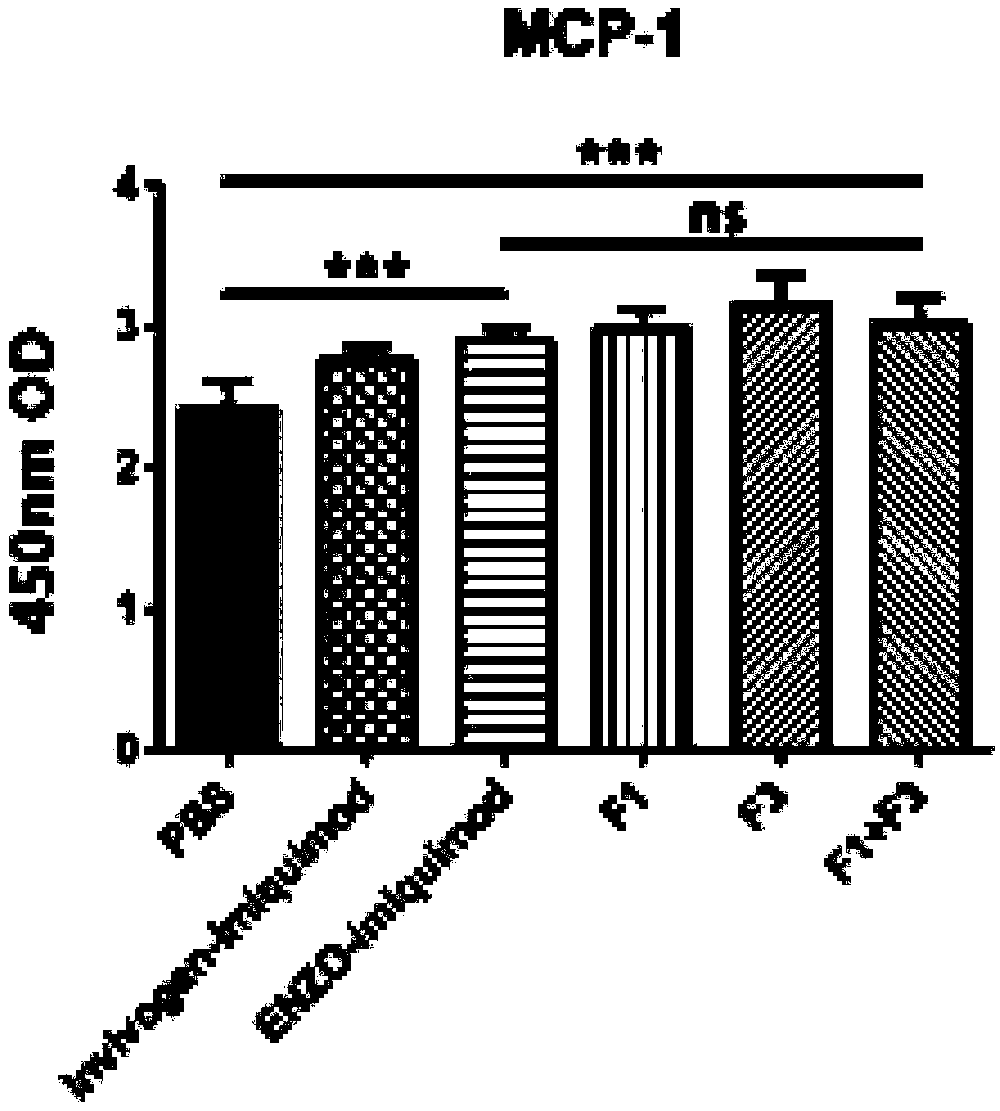
[0065] 1. Cultured in 100 microliters of 5X10 in RPMI medium containing 10% calf serum 3 TC-1 cells were added to a 96-well cell culture plate, and 5 micrograms per milliliter of F1 or / and F3 and control polypeptide P3 were added, and heated at 37°C with 5% CO 2 After culturing overnight, the supernatant was taken, and MCP-1 was detected by ELISA. F1 and F3 compositions can stimulate TC-1 cells to secrete more MCP1, the results can be found in figure 2 .
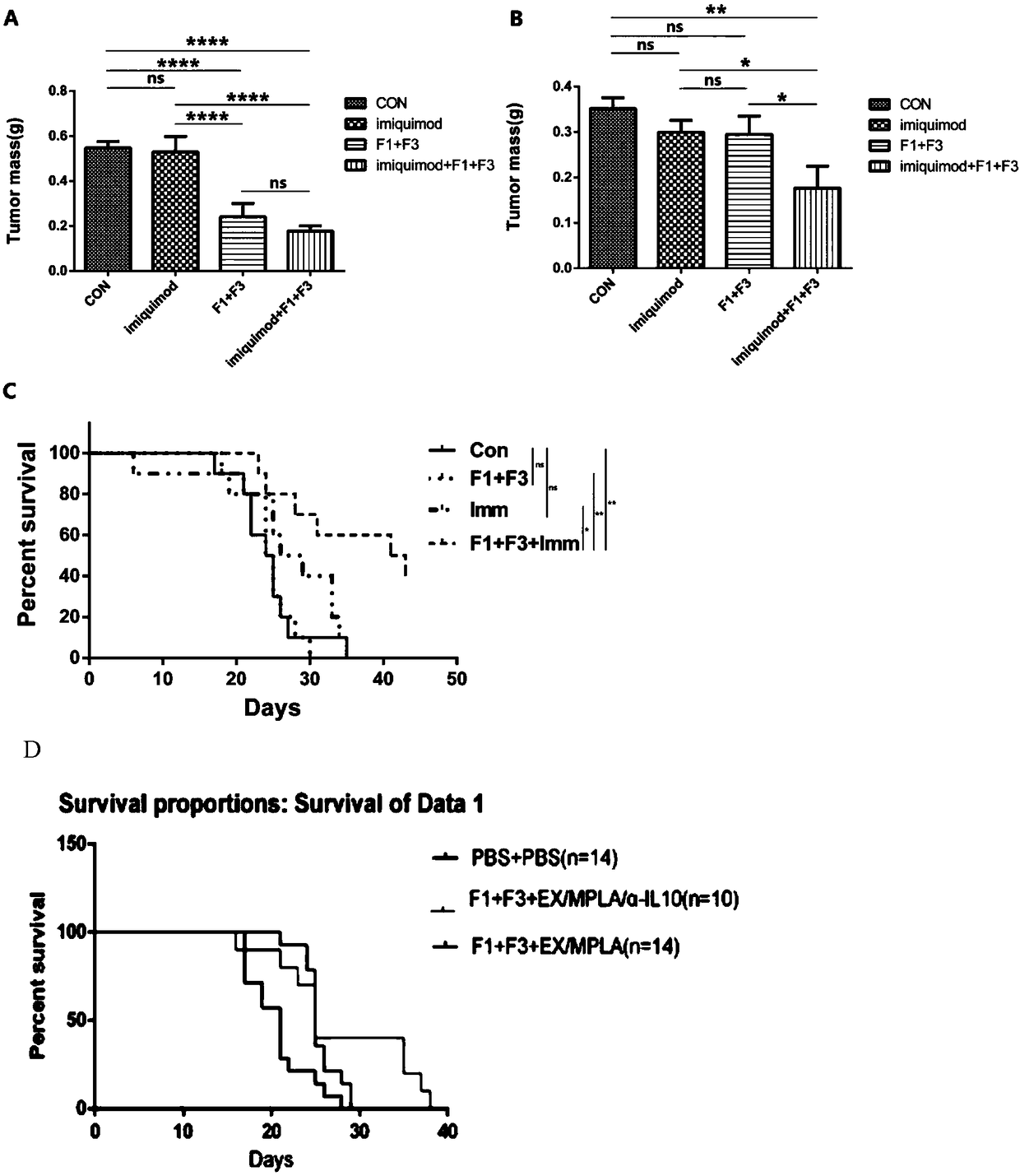
[0066] 2. Inoculate TC-1 tumor subcutaneously into C57BL / 6 mice or nude mice. After the tumor can be touched, inject F1 and F3 compositions into the tumor 7 times, 30 μg / time, Imiquimod 50 μg / time, and kill the mice two days later. Rats were isolated and weighed. Compositions F1 and F3 can inhibit the growth of TC-1 tumor in mice....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com