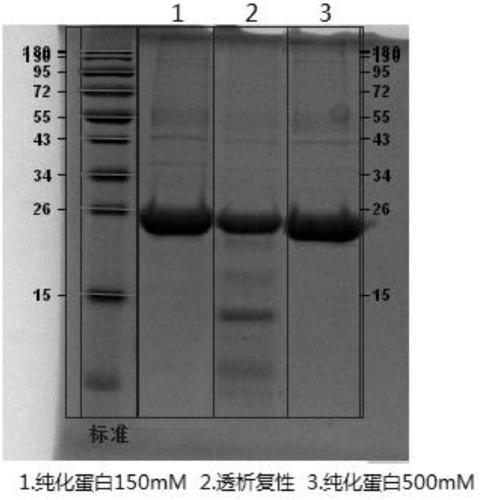
Production method of porcine foot-and-mouth disease type O genetically engineered composite epitope protein vaccine
A genetic engineering, swine foot-and-mouth disease technology, applied in the field of immunization, can solve the problems of small unit output, high production cost, unsuitable for industrial production, etc., and achieve the effects of short production cycle, fewer personnel, and less reagent consumption.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Engineering bacteria culture and induced expression:
[0034] Take 500 mL of engineered bacteria seed liquid cultivated overnight and inoculate it into 50 L of LB culture liquid (containing 75 μg / mL kanamycin), ferment at 37 ° C, pH 7.2, dissolved oxygen 40%, and add it by variable index flow feeding method; after 5 hours of fermentation, IPTG was added to a final concentration of 1.0 mmol / L, and the expression was induced for 5 hours to obtain the recombinant protein after induction.
[0035] Bacteria collection and crushing:
[0036] The fermented broth collected was concentrated using a 750KD hollow fiber column, and stopped when it reached 5L. Using a high-pressure homogenizer, the concentrated solution is crushed under the conditions of 900 bar and two cycles.
[0037] Inclusion body washing and denaturation:
[0038] Use a 750KD hollow fiber column to wash the broken fermentation broth. The buffer is 1×IBWashBuffer (EDTA 10mmol / L; Tris-HCl 20mmol / L, pH7.5; Trit...
Embodiment 2
[0044] Engineering bacteria culture and induced expression:
[0045] Take 400mL of engineered bacteria seed solution cultivated overnight and inoculate it into 50L of LB culture solution (containing 75μg / mL kanamycin), ferment at 37°C, pH7. feeding method; after 5 hours of fermentation, IPTG was added to a final concentration of 1.0 mmol / L, and the expression was induced for 5 hours to obtain the recombinant protein after induction.
[0046] Bacteria collection and crushing:
[0047] The fermented broth collected was concentrated using a 750KD hollow fiber column, and stopped when it reached 5L. Using a high-pressure homogenizer, the concentrated solution is crushed under the conditions of 900 bar and two cycles.
[0048] Inclusion body washing and denaturation:
[0049] Use a 750KD hollow fiber column to wash the broken fermentation broth. The buffer is 1×IBWashBuffer (EDTA 10mmol / L; Tris-HCl 20mmol / L, pH7.5; TritonX-1001%) in an ice bath, and equal volume replacement 5 t...
Embodiment 3
[0055] Engineering bacteria culture and induced expression:
[0056] Take 300mL of engineered bacteria seed solution cultivated overnight and inoculate it into 50L of LB culture solution (containing 75μg / mL kanamycin), ferment at 37°C, pH7. feeding method; after 5 hours of fermentation, IPTG was added to a final concentration of 1.0 mmol / L, and the expression was induced for 5 hours to obtain the recombinant protein after induction.
[0057] Bacteria collection and crushing:
[0058] The fermented broth collected was concentrated using a 750KD hollow fiber column, and stopped when it reached 5L. Using a high-pressure homogenizer, the concentrated solution is crushed under the conditions of 900 bar and two cycles.
[0059] Inclusion body washing and denaturation:
[0060] Use a 750KD hollow fiber column to wash the broken fermentation broth. The buffer is 1×IBWashBuffer (EDTA 10mmol / L; Tris-HCl 20mmol / L, pH7.5; TritonX-1001%) in an ice bath, and equal volume replacement 5 t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com