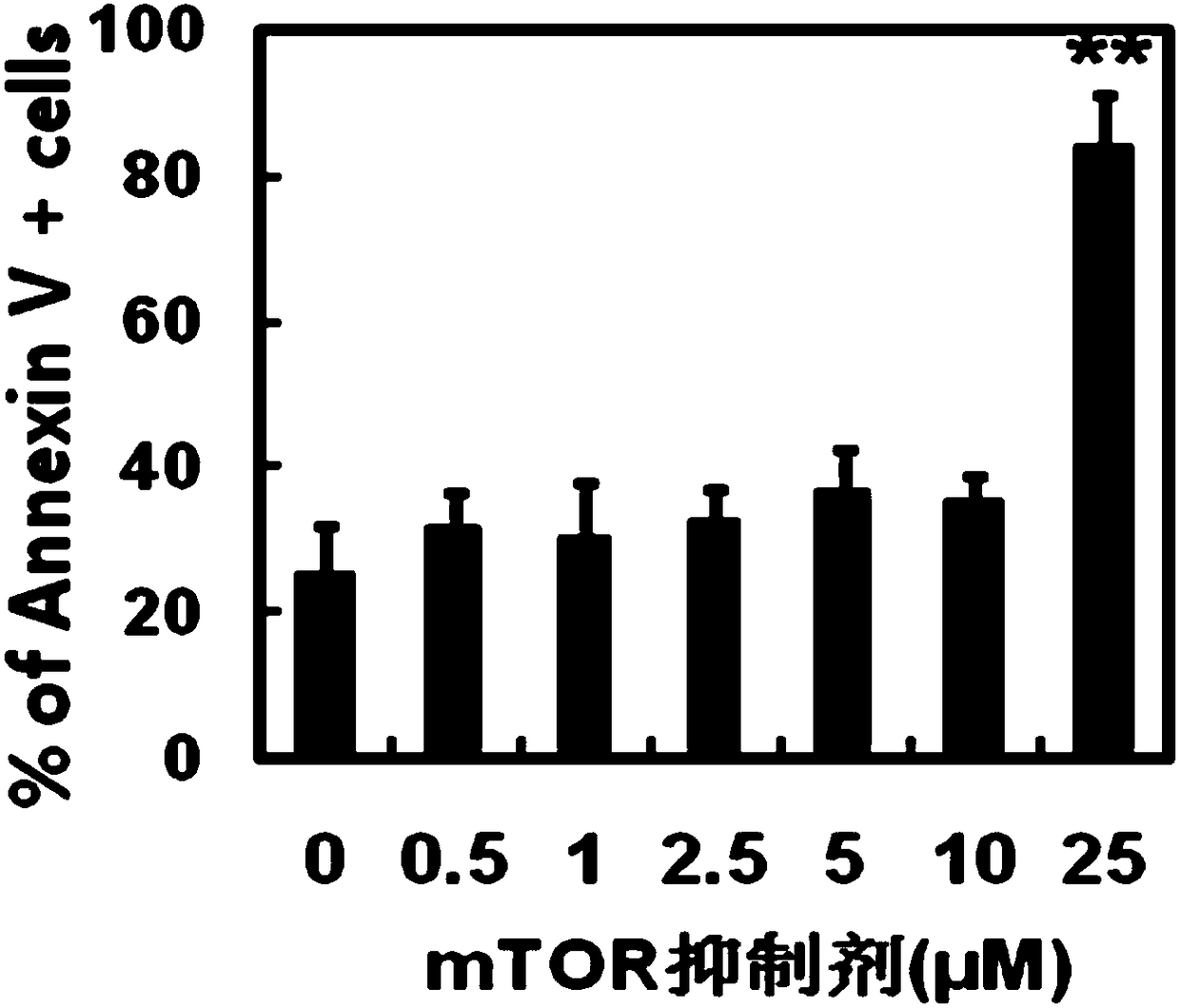
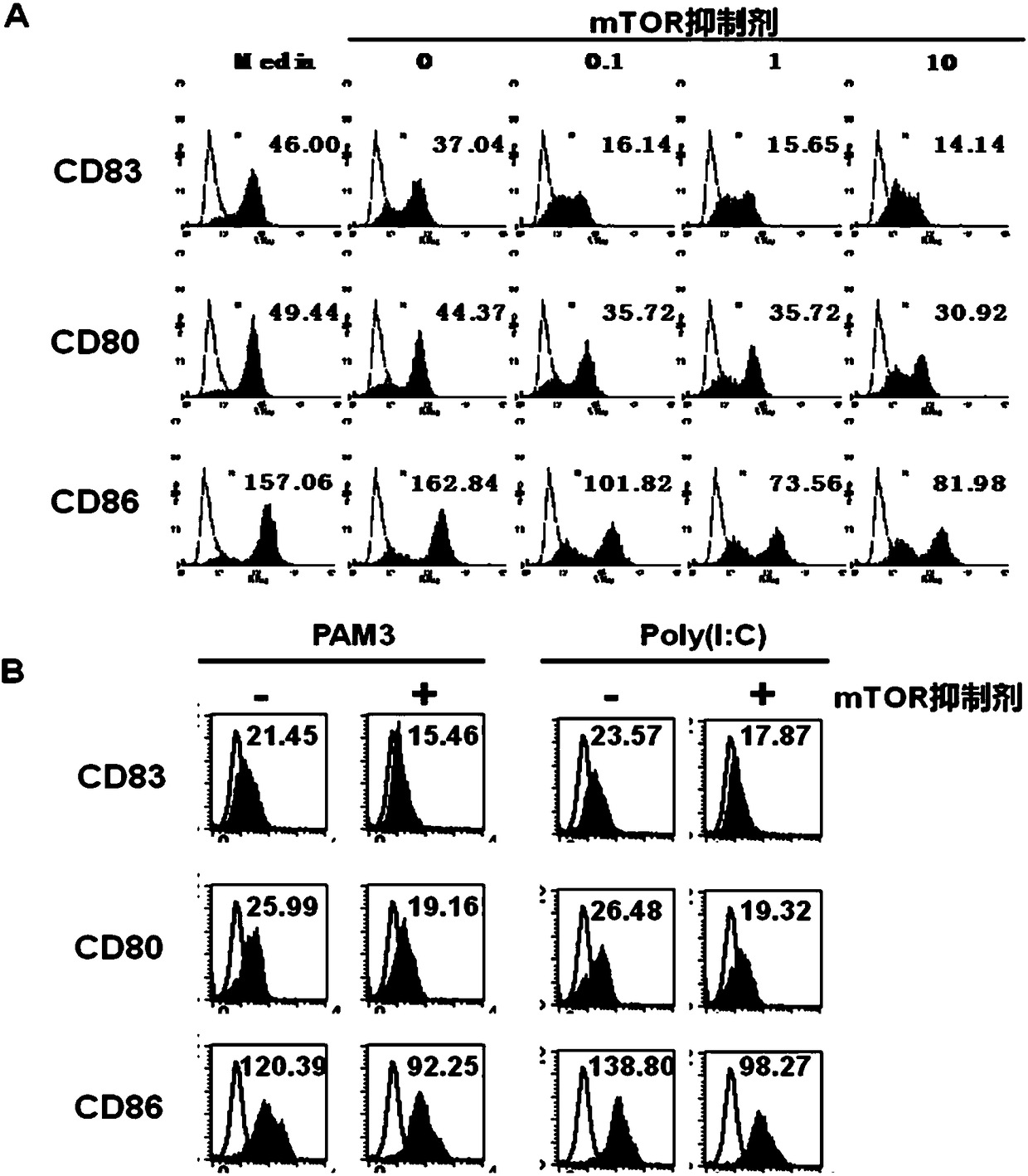
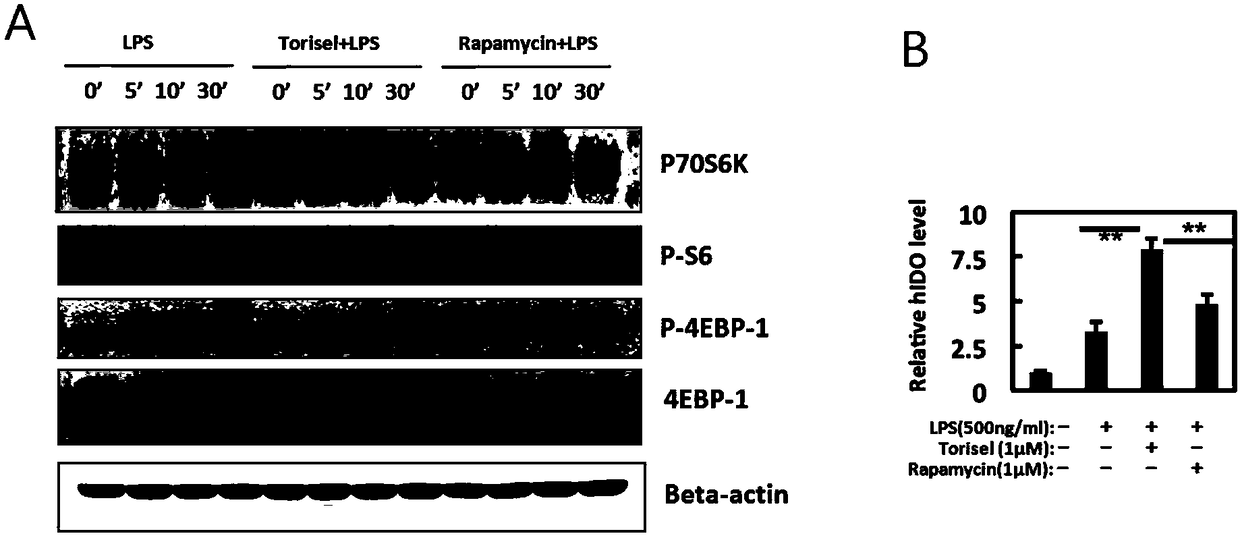
Preparation method and application of tolerogenic dendritic cells capable of keeping stability in inflammatory environment
A dendritic cell and tolerance technology, which is applied in the field of preparation of tolerance dendritic cells, can solve the problems of increased preparation cost, time-consuming and labor-intensive, and difficult preservation of cytokines, and achieves stable and stable preparation of finished products. Simple process, high-purity preparation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1. Materials
[0049] 1. Purified human immature dendritic cells (imDC).
[0050] 2. A small molecular compound with mTOR signaling inhibitory ability.
[0051] 3. Complete cell culture medium.
[0052] 2. Method
[0053] 1. Identification of high-purity human dendritic cells.
[0054] 1.1. Immature dendritic cells were derived from purified human monocytes cultured in vitro.
[0055] 1.2. The purity and immaturity of the cells were identified by flow cytometry to ensure that the content of CD1a+CD83- cells was 80% or above.
[0056] 2. Preparation of tDC.
[0057] 2.1. The purified immature dendritic cells are suspended in the complete cell culture medium (RPMI-1640 containing 10% FBS, RPMI-1640 containing 1%-10% human AB serum) that can ensure the normal survival nutritional requirements of primary cells , and other serum-free medium suitable for DC culture).
[0058] 2.2. Adjust the cell suspension concentration to 5*10 4 / ml-5*10 6 / ml.
[0059] 2.3. Add s...
Embodiment 2
[0088] Purified immature dendritic cells were suspended in RPMI-1640 medium containing 10% FBS, and the concentration of the cell suspension was adjusted to 5*10 4 / ml-5*10 6 / ml. Add Rapamycin to the culture system, adjust the final concentration of Rapamycin to 5 μM, and place at 37°C, 5% CO 2 Incubate for 4 hours under culture conditions with saturated humidity. DNFB activation was added to i.v. infused mice of the same type. At the same time, the DNFB control group was set up, and the immune initialization was induced for 5 days. After DNFB was smeared on the mouse ears, treated for 48 hours, the ears were taken for immunohistochemical and staining analysis, and the degree of control of tDC cell products on atopic dermatitis was analyzed by the degree of ear swelling. The results showed that the degree of ear swelling in the DNFB+Rapamycin group was 48% lower than that in the DNFB control group.
Embodiment 3
[0090] Purified immature dendritic cells were suspended in RPMI-1640 medium containing 10% FBS, and the concentration of the cell suspension was adjusted to 5*10 4 / ml-5*10 6 / ml. Add Temisirolimus to the culture system, adjust the final concentration of Temisirolimus to 10 μM, and place it at 37°C, 5% CO 2 Incubate for 6 hours under culture conditions with saturated humidity. DNFB activation was added to i.v. infused mice of the same type. At the same time, the DNFB control group was set up, and the immune initialization was induced for 5 days. After DNFB was smeared on the mouse ears, treated for 48 hours, the ears were taken for immunohistochemical and staining analysis, and the degree of control of tDC cell products on atopic dermatitis was analyzed by the degree of ear swelling. The results showed that the degree of ear swelling in the DNFB+Temisirolimus group was 46% lower than that in the DNFB control group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com