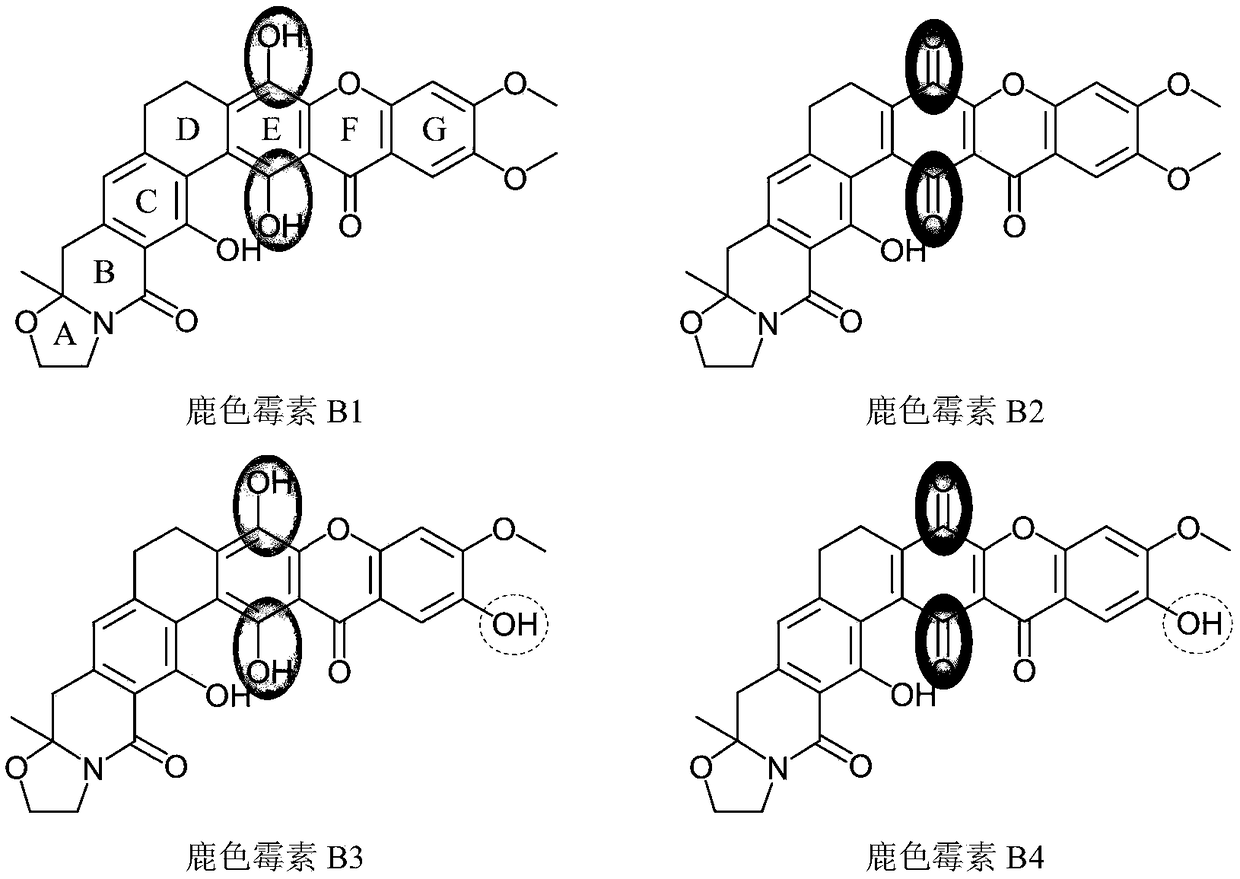
Cervinomycin B1, B2, B3 and B4 and production method and application thereof
A technology of cervitromycin and antibiotics, applied in the field of medicine and biology, can solve the problem of difficult control of drug-resistant bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Embodiment 1: Streptomyces CGMCC NO.16425 solid-state fermentation culture
[0065] (1) Preparation of fresh spore suspension: Streptomyces CPCC NO.204980 spore suspension frozen in a refrigerator at minus 70°C was melted and evenly spread and inoculated on 7-10 spore medium plates (medium composition: starch 20.0 g / L, soybean cake powder 20.0g / L, agar powder 15.0g / L, pH7.0; plate diameter 9.0cm, each plate is filled with about 20 ml of medium). When cultured at 28°C for 8-9 days, a layer of gray spores grew on the surface of the plate medium. Wash the spores with 8.0-10.0ml sterile water on each plate, and obtain a concentration of about 1×10 after shaking. 8 Spores / ml of spore suspension for inoculation of fermentation medium plates.
[0066] (2) Solid-state fermentation culture: evenly spread the spore suspension on the fermentation medium plate (substrate composition: cornstarch 10.0g / L, cottonseed cake powder 10.0g / L, threonine 10.0g / L, agar powder 15.0 g / L, pH ...
Embodiment 2
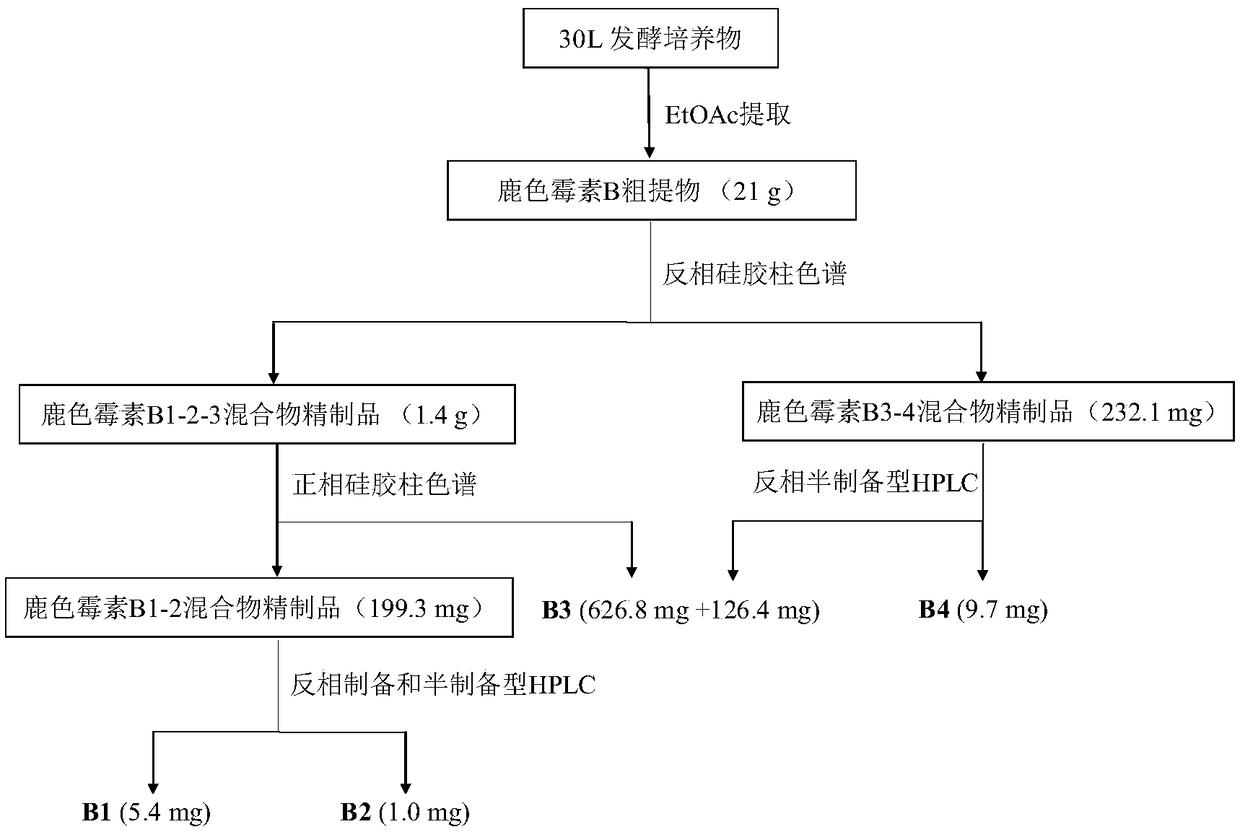
[0067] Embodiment 2: Separation and purification of cercomycin B component
[0068] For the separation and purification process of cercomycin B1, B2, B3, and B4 components, please refer to figure 2 .
[0069] (1) Ethyl acetate extraction :
[0070] Streptomyces CPCC NO.204980 solid-state fermentation culture (about 30L), adding an equal volume of ethyl acetate for extraction, soaking and extracting for 2 days each time, and extracting twice in total. The ethyl acetate extract was collected and evaporated in vacuo to obtain a brown ethyl acetate extract (about 21 g).
[0071] (2) Refined product of cervitromycin B component mixture (reversed-phase silica gel column for impurity removal) :
[0072] Redissolve the brown ethyl acetate extract in an appropriate amount of methanol (about 200ml), and sonicate to induce dissolution. According to the ratio of 1:1.5, weigh about 31g of reverse phase (ODS) silica gel chromatography column filler, add it to the methanol solution,...
Embodiment 3
[0096] Embodiment 3: Oxidation preparation of cercomycin B2 and B4 components
[0097] The components B2 and B4 of cercomycin are the oxidation products of components B1 and B3 of cercomycin, respectively. In embodiment 2, the yield of preparing cercomycin B2 and B4 components by separation and purification is relatively low, so the pure or semi-pure products of cercomycin B1 and B3 components obtained in embodiment 2 can be utilized. The chemical oxidation method is more efficient to prepare cercomycin B2 and B4 fractions. details as follows:
[0098] (1) Weigh 25mg (or 5mg) of cercomycin B1 (or B3) component, and dissolve it in 25.0ml (5.0ml) dichloromethane-methanol (volume ratio 1:1) by ultrasonic; weigh 25mg (or 5 mg) of silver oxide was added to the solution and stirred at room temperature.
[0099] (2) Take 100 μl of the supernatant of the reaction solution every 1 hour, and filter it with a 0.22 μm filter membrane to remove silver oxide; after removing the solvent i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com