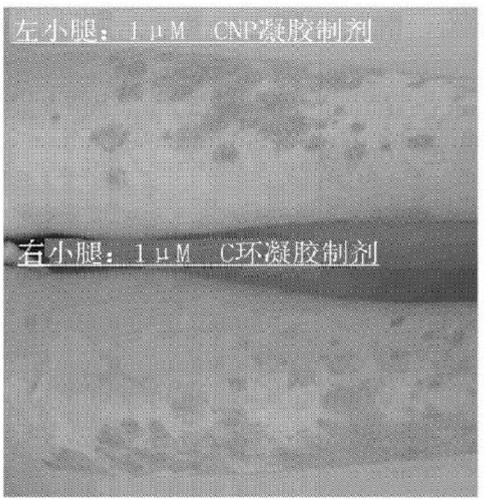
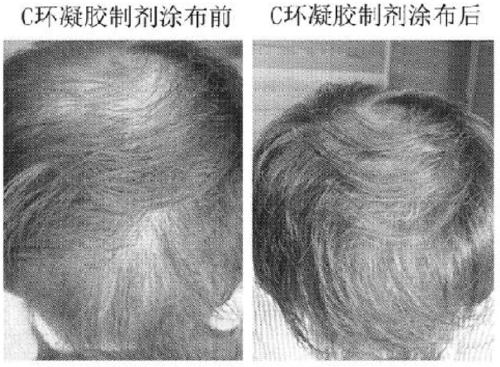
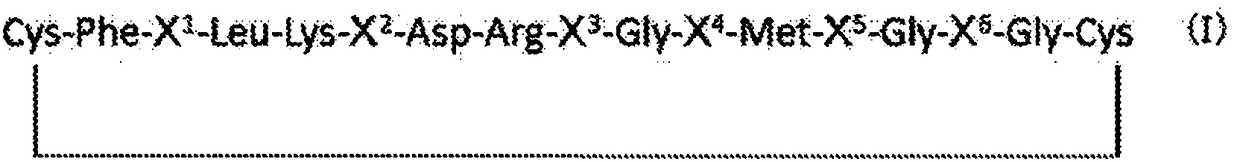Cnp cyclic peptide, and medicine, external preparation and cosmetic each containing said cyclic peptide
A technology for external preparations and cosmetics, which is applied in the fields of CNP cyclic peptides and drugs, external preparations and cosmetics containing the cyclic peptides, can solve the problems of long duration of effects and short therapeutic effects, and achieves rough skin improvement, excellent quick-acting properties, long relief effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0292] The present invention will be described in more detail through examples below. However, the present invention is not limited by these Examples.
[0293] 1. Manufacture of Cyclic Peptides
[0294]Firstly, a cyclic peptide formed by the amino acid sequence shown in formula I is synthesized.
[0295] Specifically, using a peptide synthesizer, amino acids were sequentially combined by a peptide solid-phase synthesis method to form a linear peptide consisting of 17 amino acids. Then, the protecting groups of the first Cys and the 17th Cys from the N-terminus were removed. After that, iodine (I 2 ) treatment to oxidatively form cysteine bonds between amino acid residues to form cyclic peptides.
[0296] The obtained cyclic peptide-containing composition was purified by reverse-phase liquid high-speed chromatography (reverse-phase HPLC), and then freeze-dried to obtain a purified cyclic peptide in the form of white powder.
[0297] In addition, mass analysis of the obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com