Application of emodin and combination of emodin and polydatin in treating metabolic syndrome
A technology for metabolic syndrome and emodin, which is applied in the application field of emodin and the combination of emodin and polydatin in the treatment of metabolic syndrome, and can solve the problems of no internal connection, single animal model factors, and lack of dynamic observation of detection indicators, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Emodin group, and the influence of emodin and Polygonum cuspidatum group on spontaneous type 2 diabetes mouse combined dyslipidemia model
[0035] Experimental materials and methods
[0036] 1. Animal model:
[0037] Spontaneous type 2 diabetic mice (KK-ay mice) combined with abnormal blood lipid metabolism model, SPF grade male 8-9 weeks old, body weight 30.94±1.67g, purchased from Beijing Huafukang Experimental Animal Technology Co., Ltd., license number SCXK (Beijing) 2009-0004. Six male C57BL / 6J mice, weighing 24.57±1.60 g, were purchased from Beijing Huafukang Experimental Animal Technology Co., Ltd., license number SCXK (Beijing) 2009-0007.
[0038] 2. Test drug:
[0039] Emodin: National drug standard substance, about 20mg package, batch number: 110756-200110 (National Institute for Food and Drug Control)
[0040] Polydatin: chemical reference substance, about 20mg package, batch number: 111575-200502 (National Institute for Food and Drug Cont...
Embodiment 2
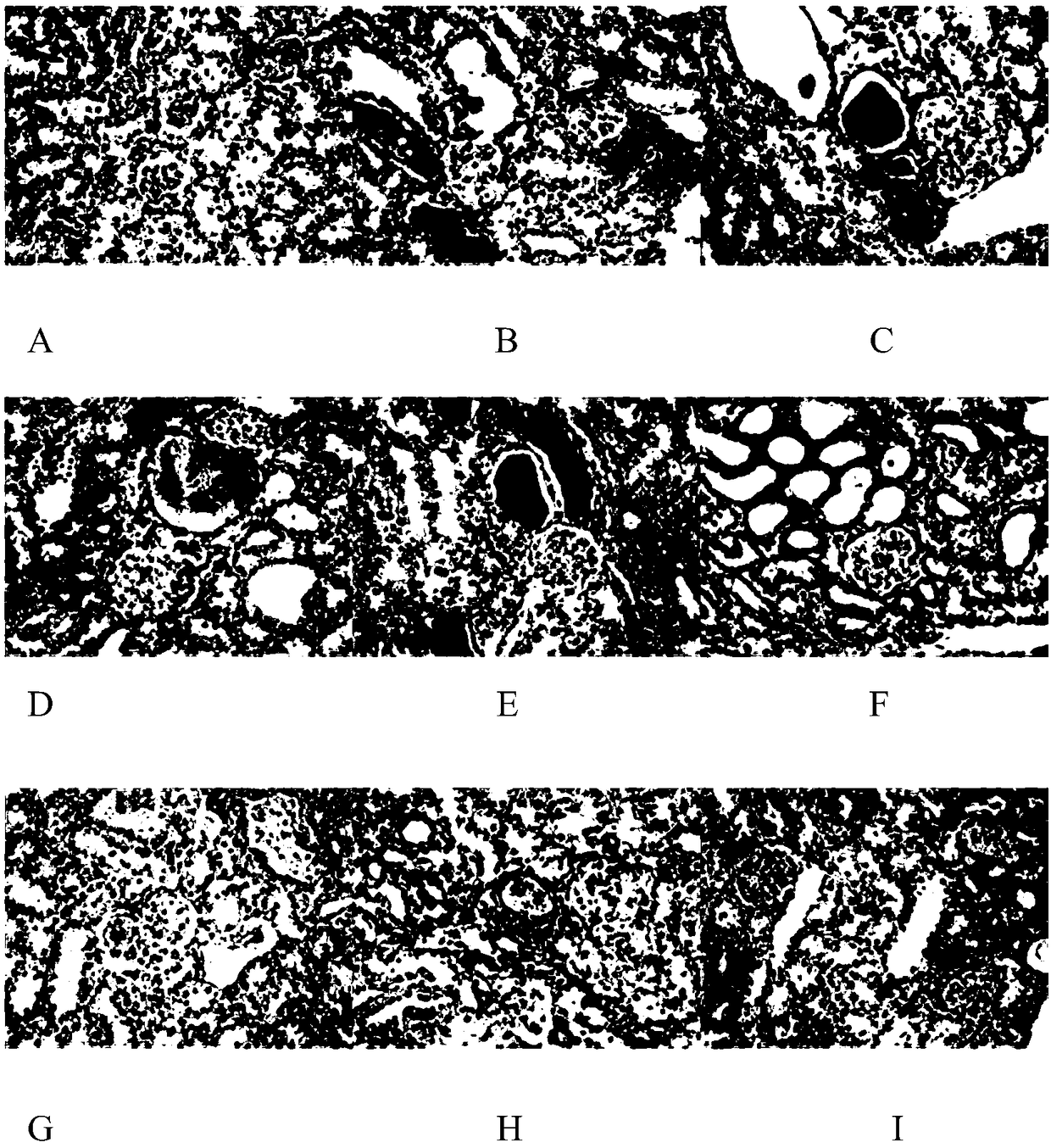
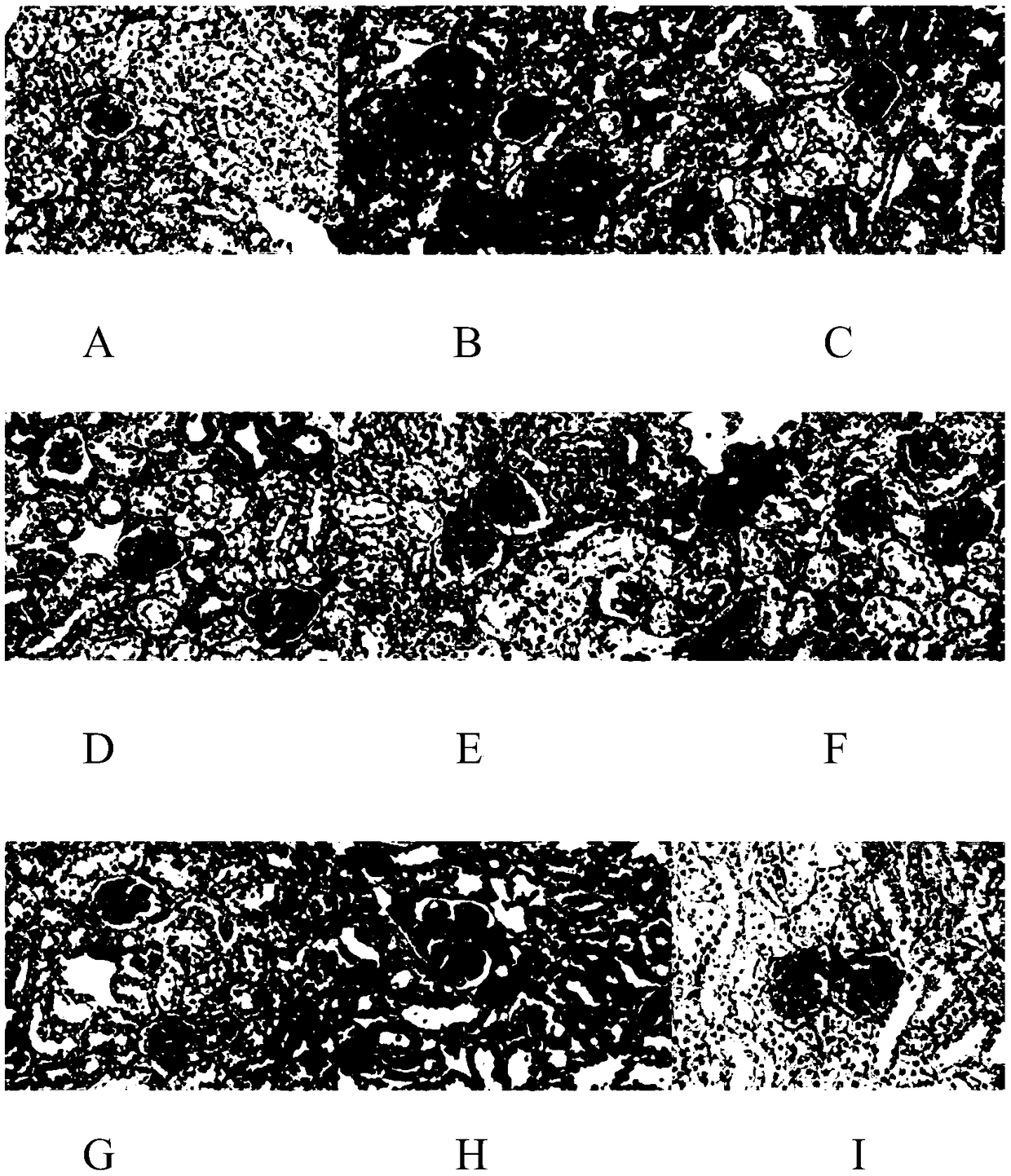
[0076] Embodiment 2: the influence of emodin and emodin+polydatin on KK-ay mouse kidney histopathological changes:
[0077] 1. HE staining
[0078] In the model control group, glomerular lesions, renal tubular lesions, renal interstitial inflammatory infiltration, and the total score of lesions were all significantly increased, compared with the normal control group (P<0.1); emodin and emodin+polydatin were continuously After 12 weeks of administration, the glomerular lesions, renal tubular lesions, and renal interstitial inflammatory infiltration can be reduced to varying degrees within the tested dosage range, and the total lesion score can be reduced, which is significantly different from that of the model control group (P<0.1 ).
[0079] Table 5 Classification score of renal lesions in each group of emodin and emodin+polydatin
[0080]
[0081] Note: Compared with the normal group, #P<0.05; compared with the model group: *P<0.05.
[0082] Table 6 The total score of kid...
Embodiment 3
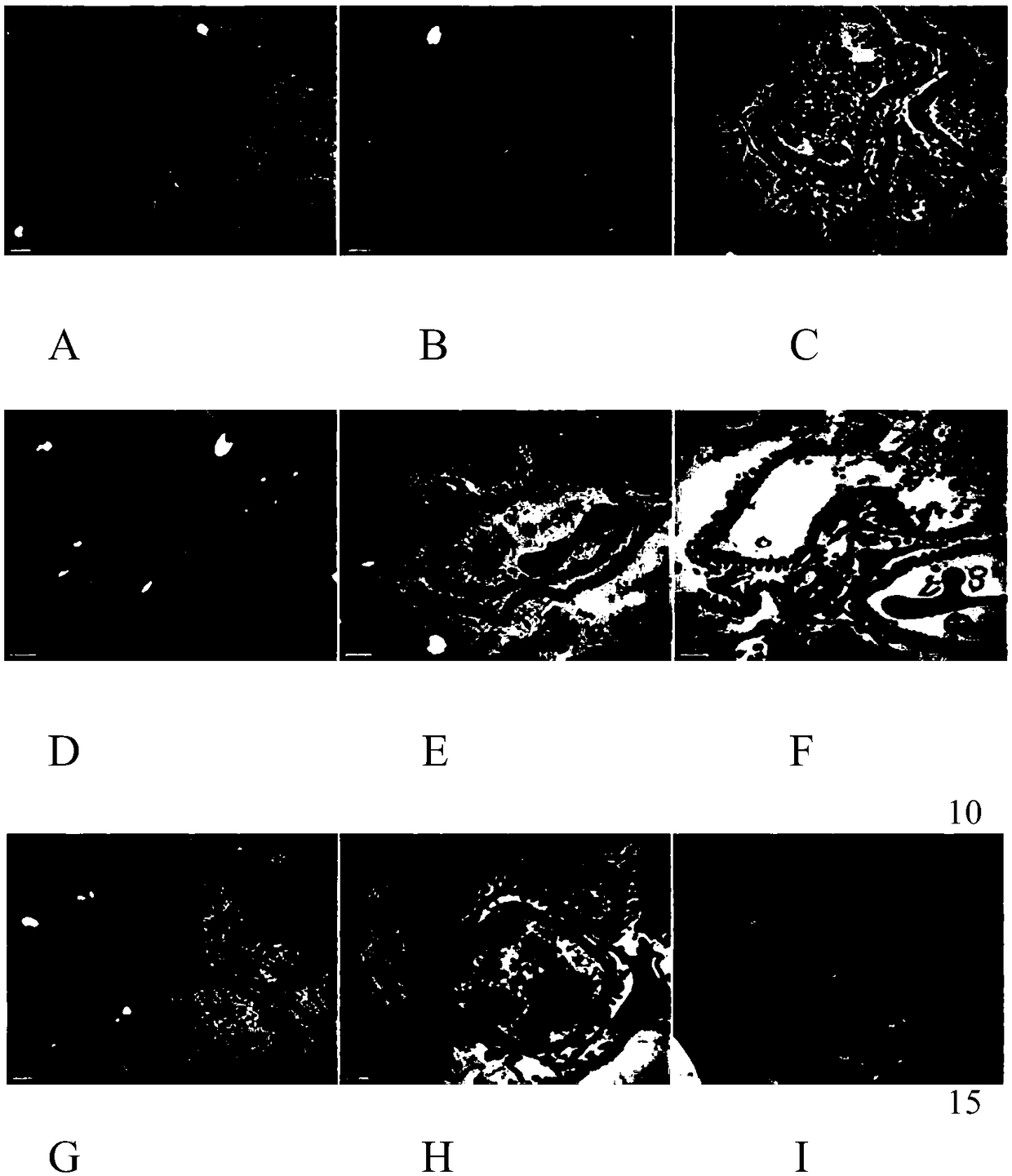
[0092] Example 3: Effects of emodin and emodin+polydatin on AMPK / iNOS / NF-κB signaling pathway in spontaneous type 2 diabetic mice with dyslipidemia model
[0093] 1. Tested drug: Emodin: National drug standard substance, about 20mg package, batch number: 110756-200110 (National Institute for Food and Drug Control)
[0094] Polydatin: chemical reference substance, about 20mg package, batch number: 111575-200502 (National Institute for Food and Drug Control)
[0095] Dose design:
[0096] High, medium and low doses of emodin: use distilled water to prepare large, medium and small doses of 13.33mg / (kg·d), 6.67mg / (kg·d), 3.33mg / (kg·d) respectively
[0097] High, medium and low doses of emodin + polydatin: take half the weight of emodin and polydatin respectively and prepare 13.33mg / (kg·d), 6.67mg / (kg·d), 3.33mg / (kg·d) with distilled water large, medium and small doses
[0098] 2. Experimental animals: SPF grade male KK-Ay mice aged 8-9 weeks, weighing (30.94+1.67) g, purchased ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com