Method for preparing 3- hydroxybutyrate
A technology of hydroxybutyrate and hydroxybutyric acid, which is applied in the field of preparation of 3-hydroxybutyrate, can solve the problems of low purity and high cost of 3-hydroxybutyrate, so as to improve product yield, save material loss and Effects of energy consumption and production cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
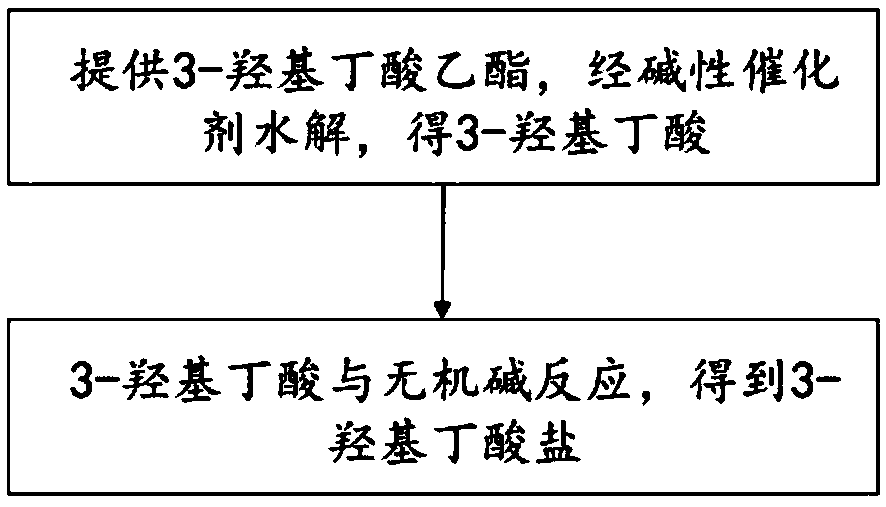
[0037] refer to figure 1 , the preparation method of the 3-hydroxybutyrate of the present embodiment, comprising:
[0038] (1) provide ethyl 3-hydroxybutyrate or methyl 3-hydroxybutyrate, and hydrolyze with a basic catalyst to obtain 3-hydroxybutyrate;
[0039] (2) 3-hydroxybutyric acid is reacted with an inorganic base to obtain 3-hydroxybutyrate.
[0040] In this embodiment, the basic catalyst is sodium hydroxide, potassium hydroxide, calcium hydroxide, magnesium hydroxide or lithium hydroxide, ethyl 3-hydroxybutyrate or methyl 3-hydroxybutyrate and alkali catalyst The molar ratio is 1:2.5~4.5. ,
[0041] In this embodiment, the molar ratio of the 3-hydroxybutyric acid to the inorganic base is 2 to 2.1:1.5, and the inorganic base is potassium hydroxide, calcium hydroxide, magnesium hydroxide or sodium hydroxide, corresponding to the obtained The 3-hydroxybutyrate is potassium 3-hydroxybutyrate, calcium 3-hydroxybutyrate, magnesium 3-hydroxybutyrate or sodium 3-hydroxybut...
no. 2 example
[0057] Add 200 grams of water into the reaction vessel, add 130 grams of ethyl 3-hydroxybutyrate or methyl 3-hydroxybutyrate under stirring to dissolve, after it is completely dissolved, add 166 grams of lithium hydroxide, and then heat up to 35°C for constant temperature reaction 24 hours.
[0058] After the reaction is completed, distill off water below 20°C, cool down to 0°C, add ethanol, fully stir and disperse for 2 hours, cool down to below 0°C to crystallize, keep warm at 0°C-5°C for 24 hours, and separate by suction filtration , washed the solid with ethanol, and dried at 35° C. to obtain 147 grams of 3-hydroxybutyric acid product, with a yield of 90.1%.
[0059] Take the finished product of 3-hydroxybutyric acid and put it into the reaction container, then add ethanol, stir to dissolve, add 105 grams of calcium hydroxide, and then raise the temperature to 45°C for 12 hours of constant temperature reaction. 0°C, add isopropanol to fully stir and disperse, cool down to...
no. 3 example
[0061] Add 300 grams of water into the reaction vessel, add 150 grams of ethyl 3-hydroxybutyrate or methyl 3-hydroxybutyrate under stirring to dissolve, after completely dissolving, add 156 grams of potassium hydroxide, and then raise the temperature to 45°C for constant temperature reaction 24 hours.
[0062] After the reaction is completed, distill off the water below 40°C, cool down to 0°C, add isopropanol, stir and disperse for 12 hours, cool down to below 0°C to crystallize, and keep warm at 0°C-5°C for 24 hours, pump After separation by filtration, the solid was washed with ethanol and dried at 55° C. to obtain 176 g of 3-hydroxybutyric acid with a yield of 95.1%.
[0063] Take the finished product of 3-hydroxybutyric acid and add it to the reaction container, then add isopropanol, stir to dissolve, add 125 grams of magnesium hydroxide, then raise the temperature to 55°C and react at a constant temperature for 12 hours. Alcohol, lower the temperature to 0°C, add ethanol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com