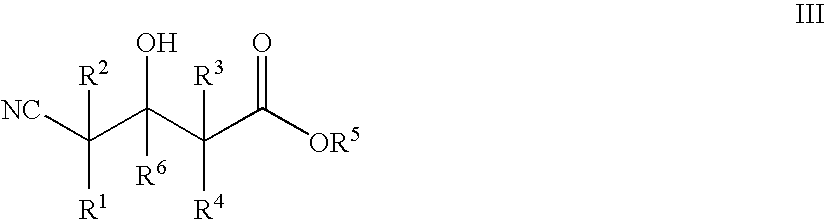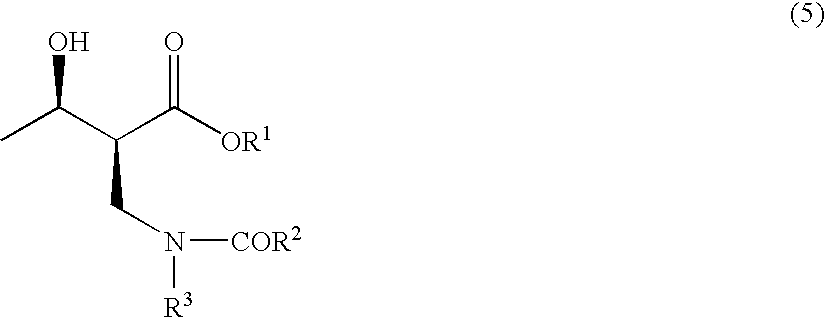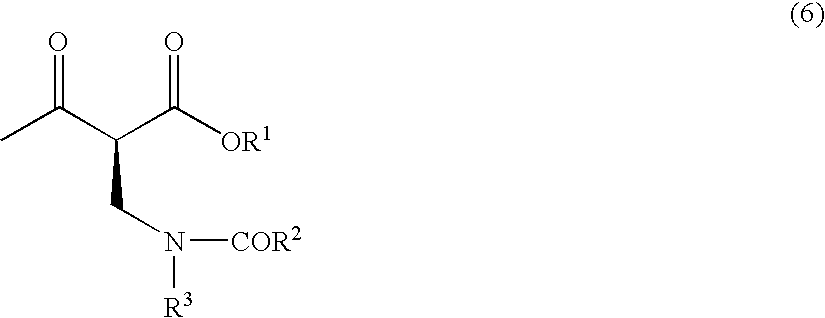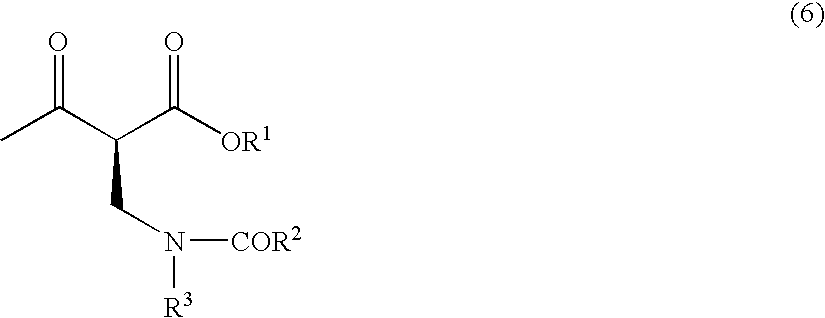Patents
Literature
93 results about "3-Hydroxybutyric Acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
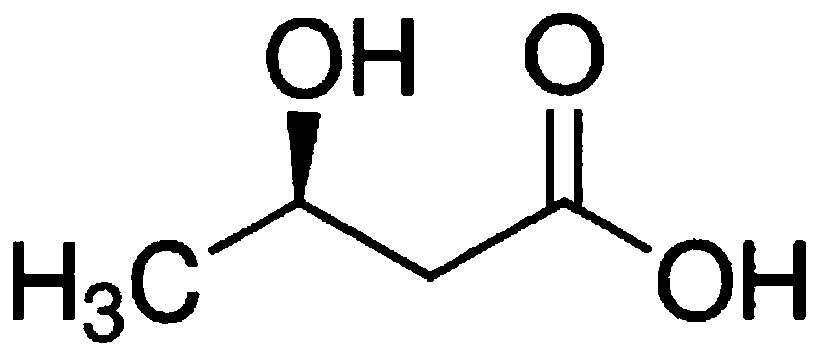
BUTYRIC ACID substituted in the beta or 3 position. It is one of the ketone bodies produced in the liver.
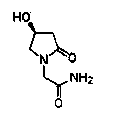
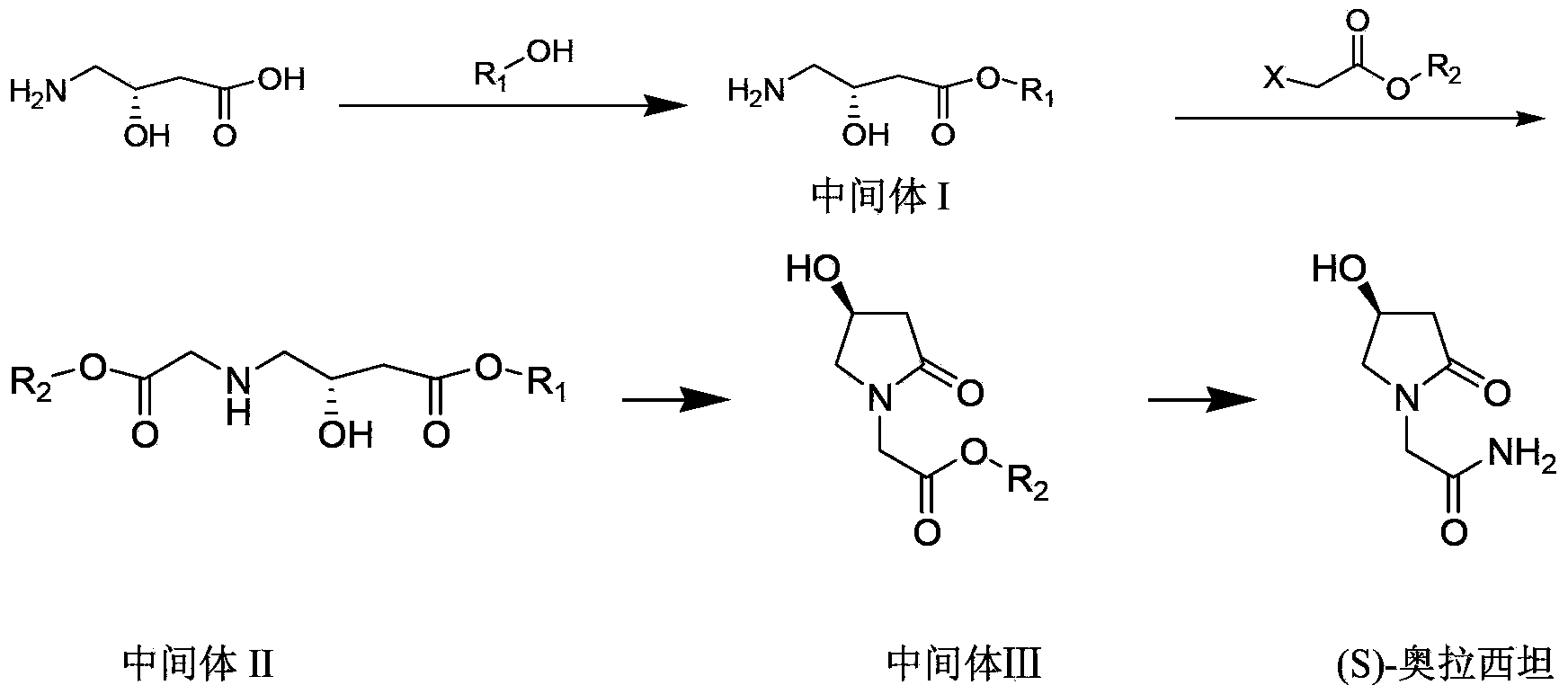
Process for the preparation of optically pure 4-hydroxy-2-oxo-1-pyrrolidine acetamide
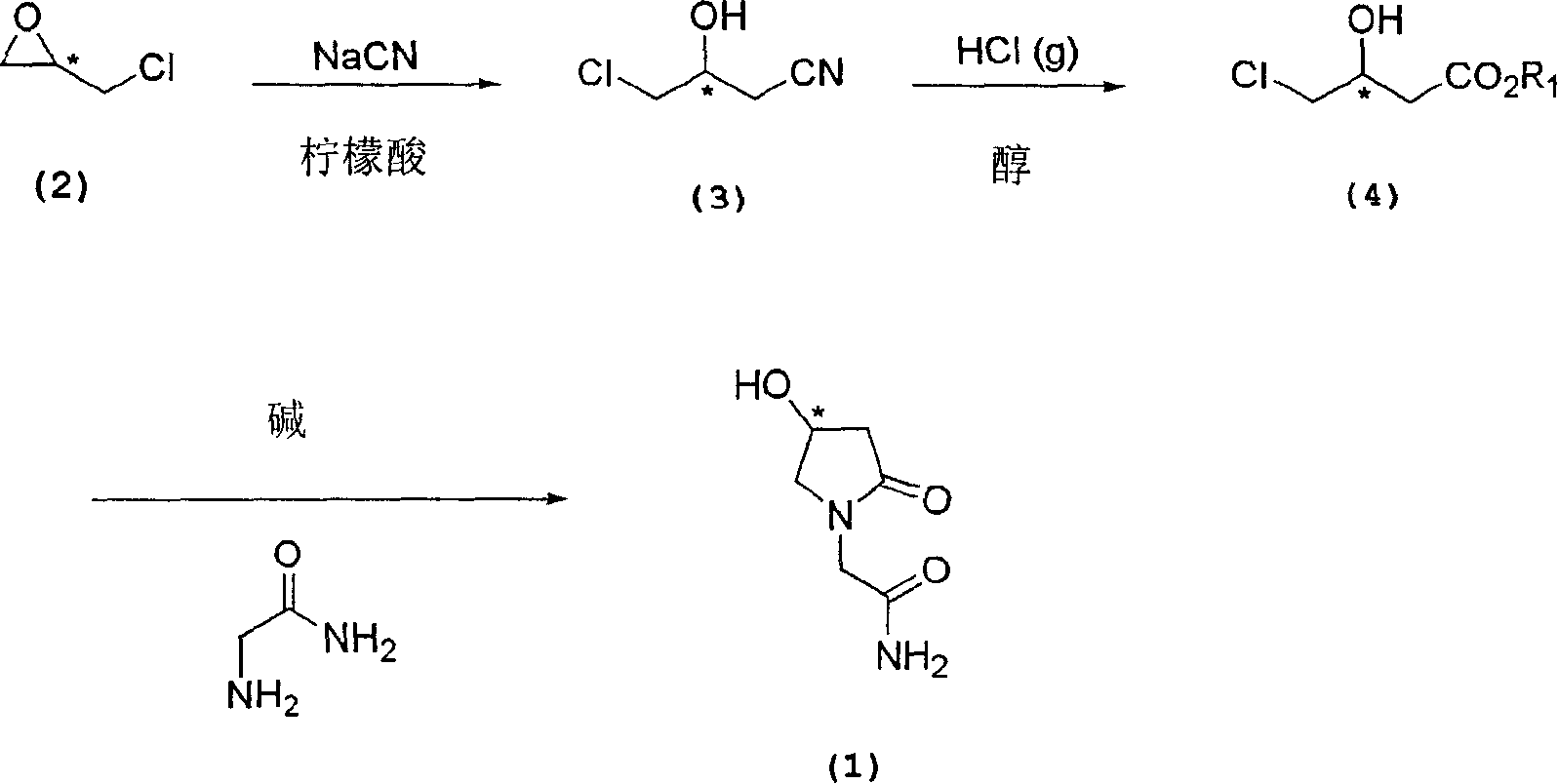
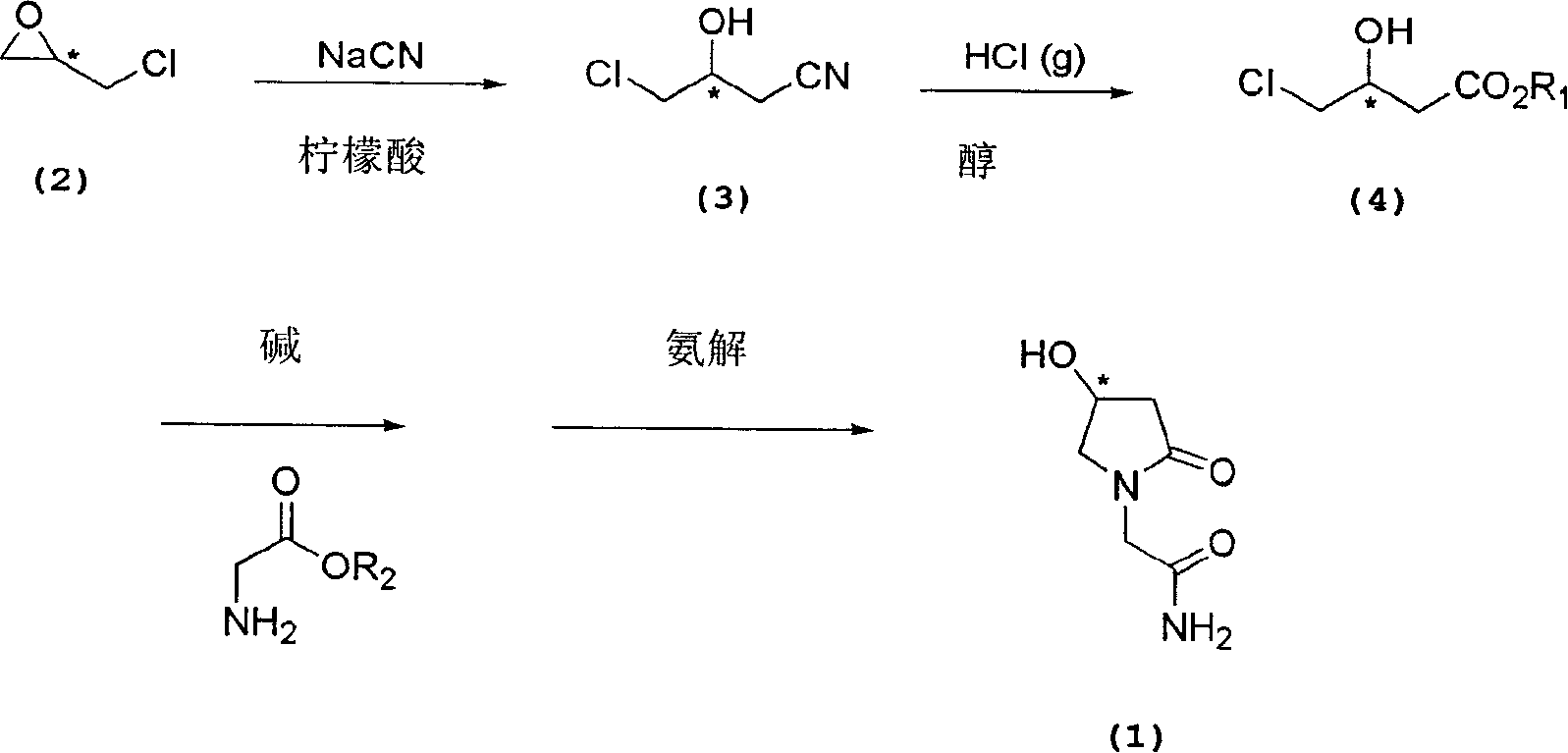
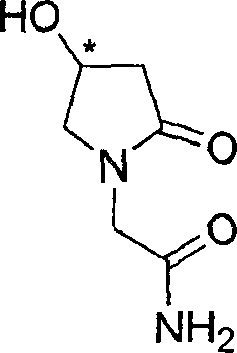
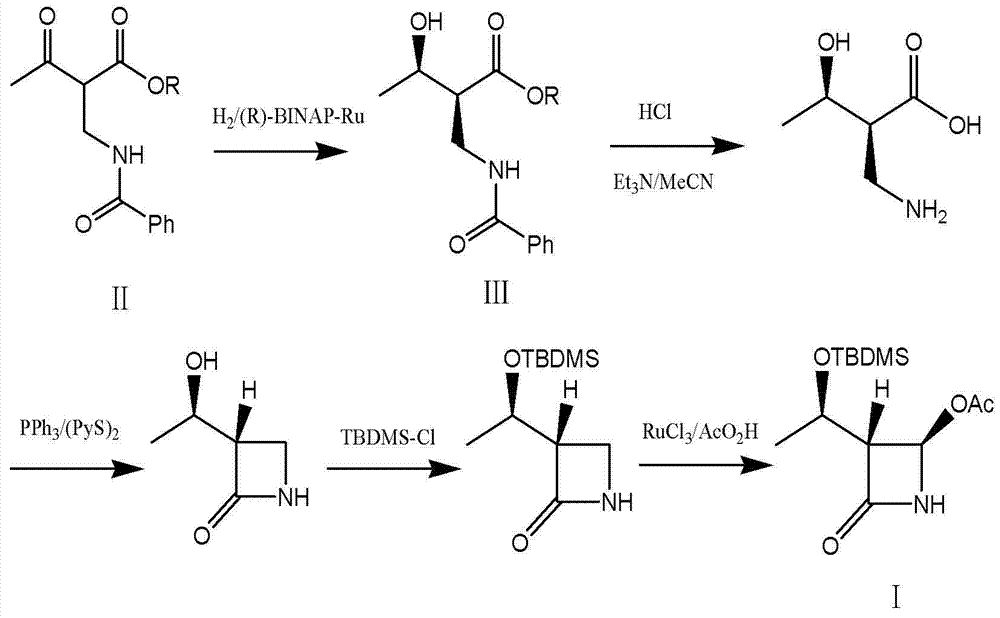
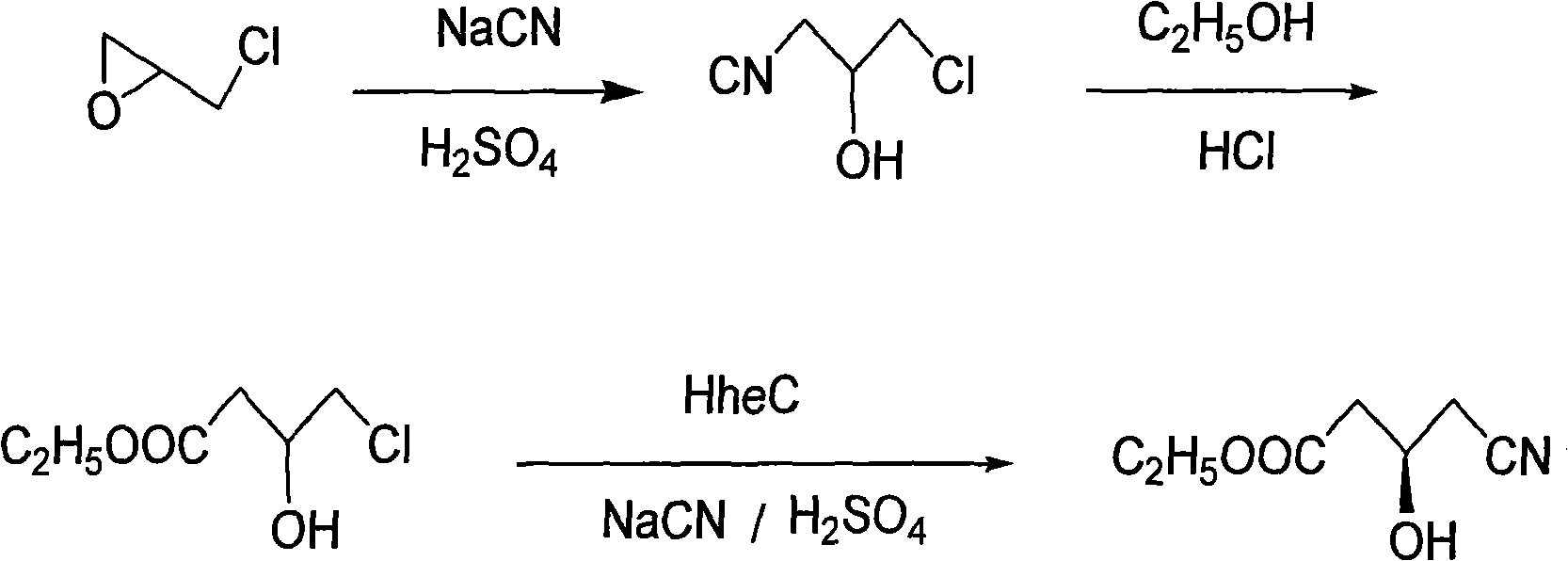
The present invention relates to a process for the preparation of chiral 4-hydroxy-2-oxo-1-pyrrolidine acetamide. The process comprises adding sodium cyanide together with citric acid to a solution of chiral epichlorohydrin to obtain chiral 3-chloro-2-hydroxypropionitrile by ring opening reaction of the chiral epichlorohydrin, reacting the obtained product with an alcohol containing hydrochloride gas to obtain chiral 4-chloro-3-hydroxybutyric acid ester, and reacting the obtained product in a presence of a base with glycinamide or with glycine ester accompanied by ammonolysis with ammonia to produce the targeted chiral 4-hydroxy-2-oxo-1-pyrrolidine acetamide. The process according to the present invention provides optically pure 4-hydroxy-2-oxo-1-pyrrolidine acetamide in high yield and in high purity, which is suitable for industrial mass-production.
Owner:AHN GOOK PHARMA CO LTD +1
Enzymatic processes for the production of 4-substituted 3-hydroxybutyric acid derivatives
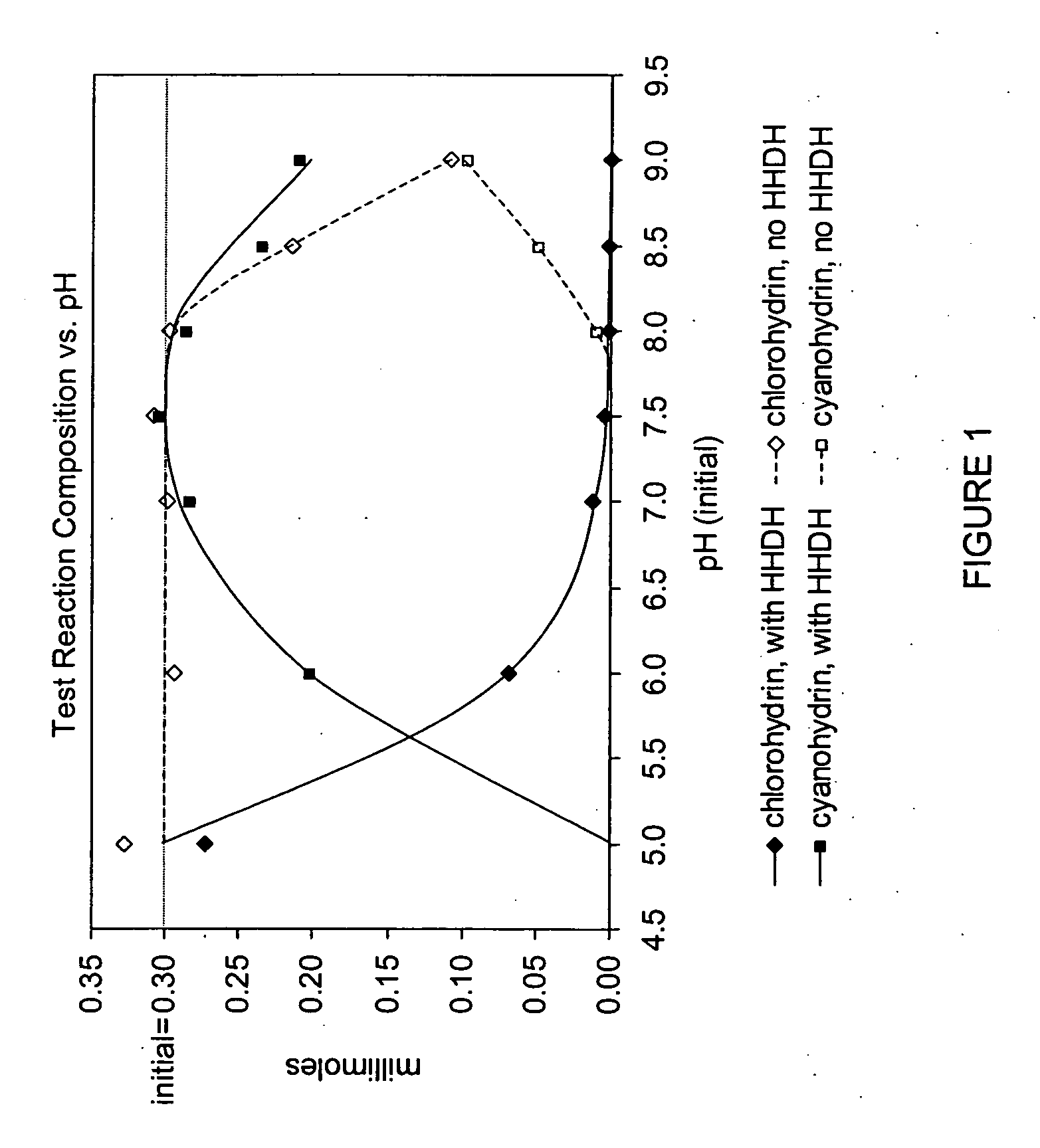
The present invention provides methods and compositions for preparing 4-substituted 3-hydroxybutyric acid derivatives by halohydrin dehalogenase-catalyzed conversion of 4-halo-3-hydroxybutyric acid derivatives. The present invention further provides methods and compositions for preparing 4-halo-3-hydroxybutyric acid derivatives by ketoreductase-catalyzed conversion of 4-halo-3-ketobutyric acid derivatives.
Owner:CODEXIS INC
Enzymatic processes for the production of 4-substituted 3-hydroxybutyric acid derivatives and vicinal cyano, hydroxy substituted carboxylic acid esters
The present invention provides methods and compositions for preparing 4-substituted 3-hydroxybutyric acid derivatives by halohydrin dehalogenase-catalyzed conversion of 4-halo-3-hydroxybutyric acid derivatives. The present invention further provides methods and compositions for preparing 4-halo-3-hydroxybutyric acid derivatives by ketoreductase-catalyzed conversion of 4-halo-3-ketobutyric acid derivatives The present invention also provides methods and compositions for preparing vicinal cyano, hydroxyl substituted carboxylic acid esters.
Owner:CODEXIS INC
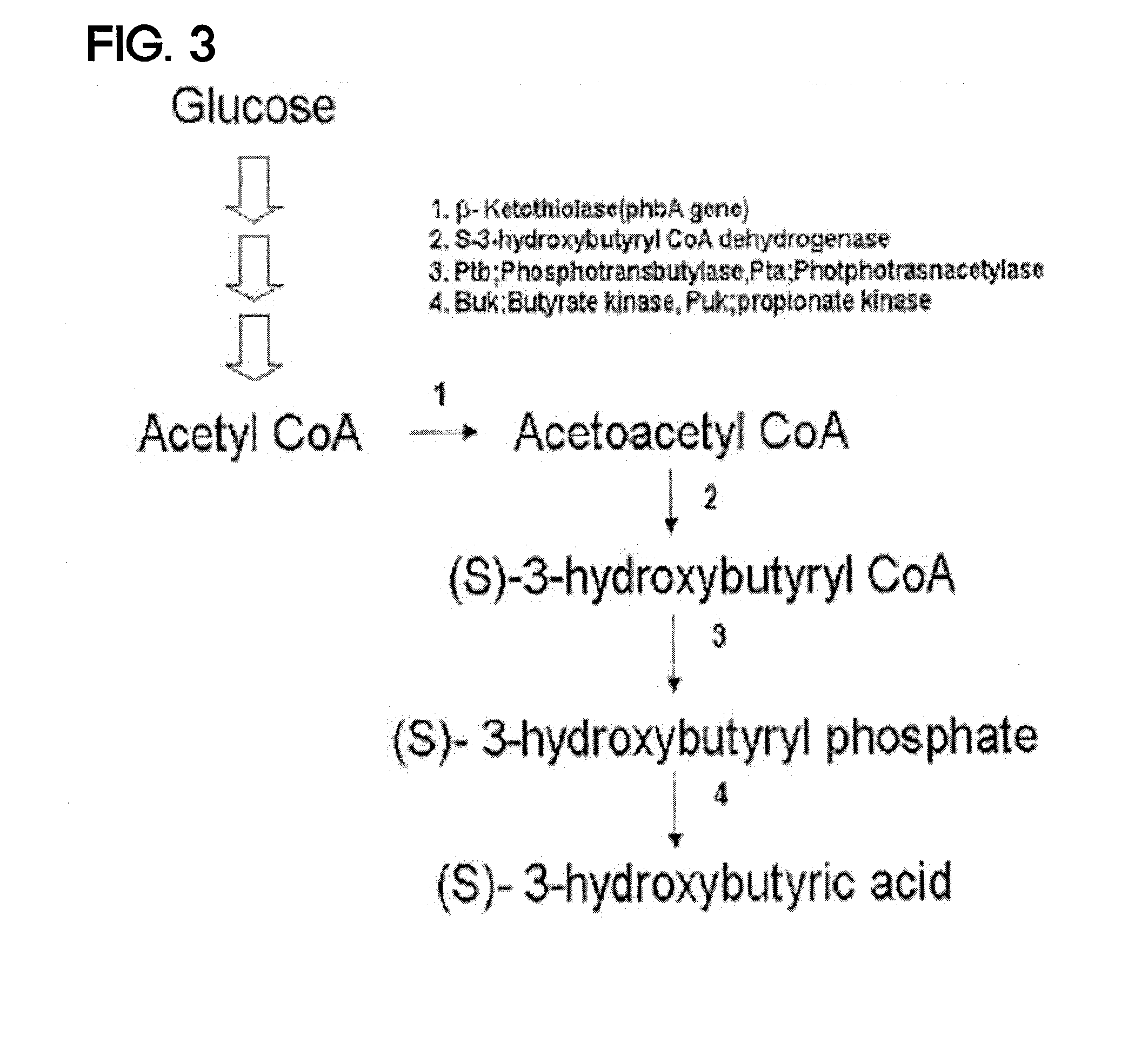
Method for the production of D-(-)-3-hydroxybutyric acid by recombinant esherichia coli
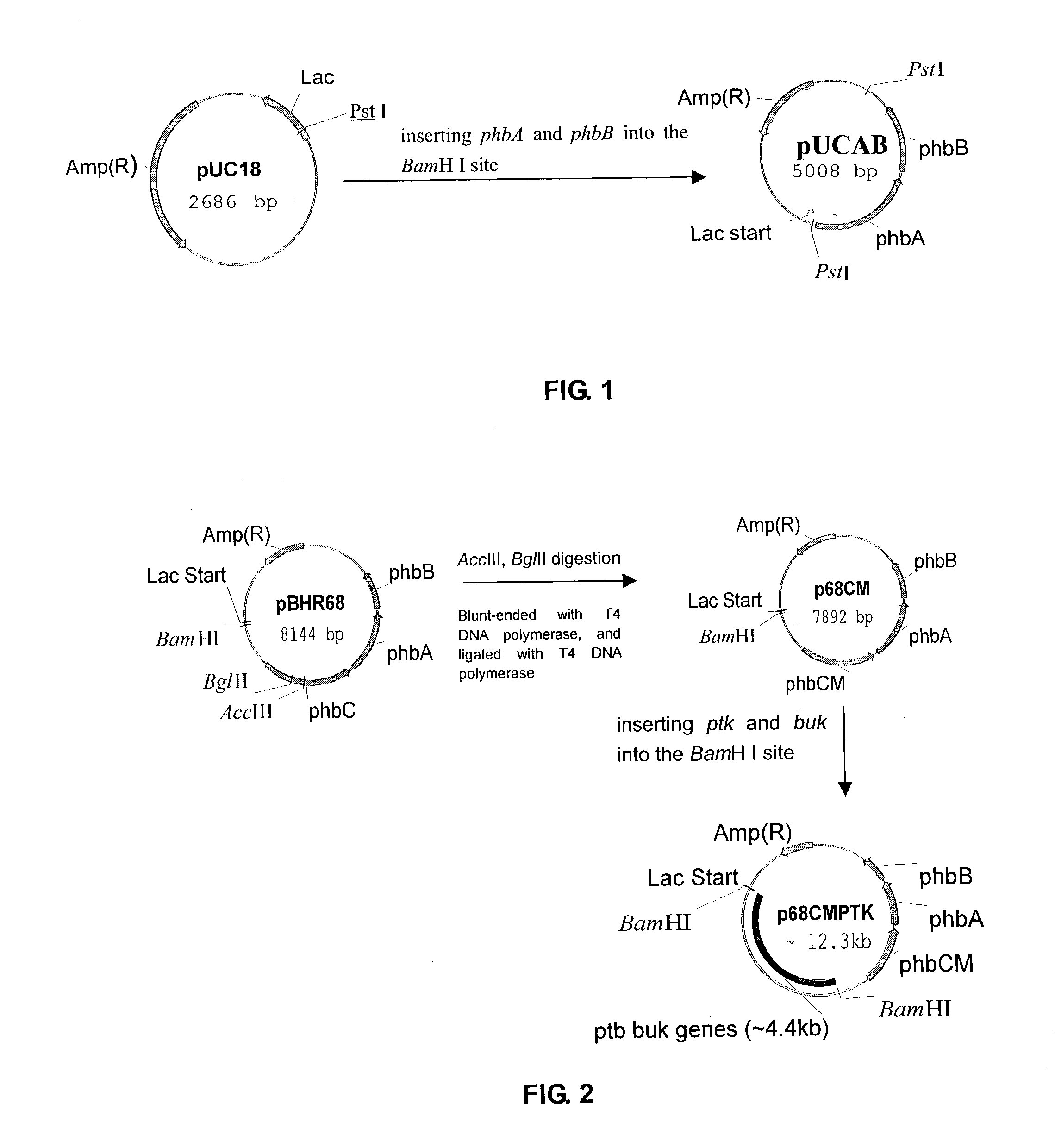
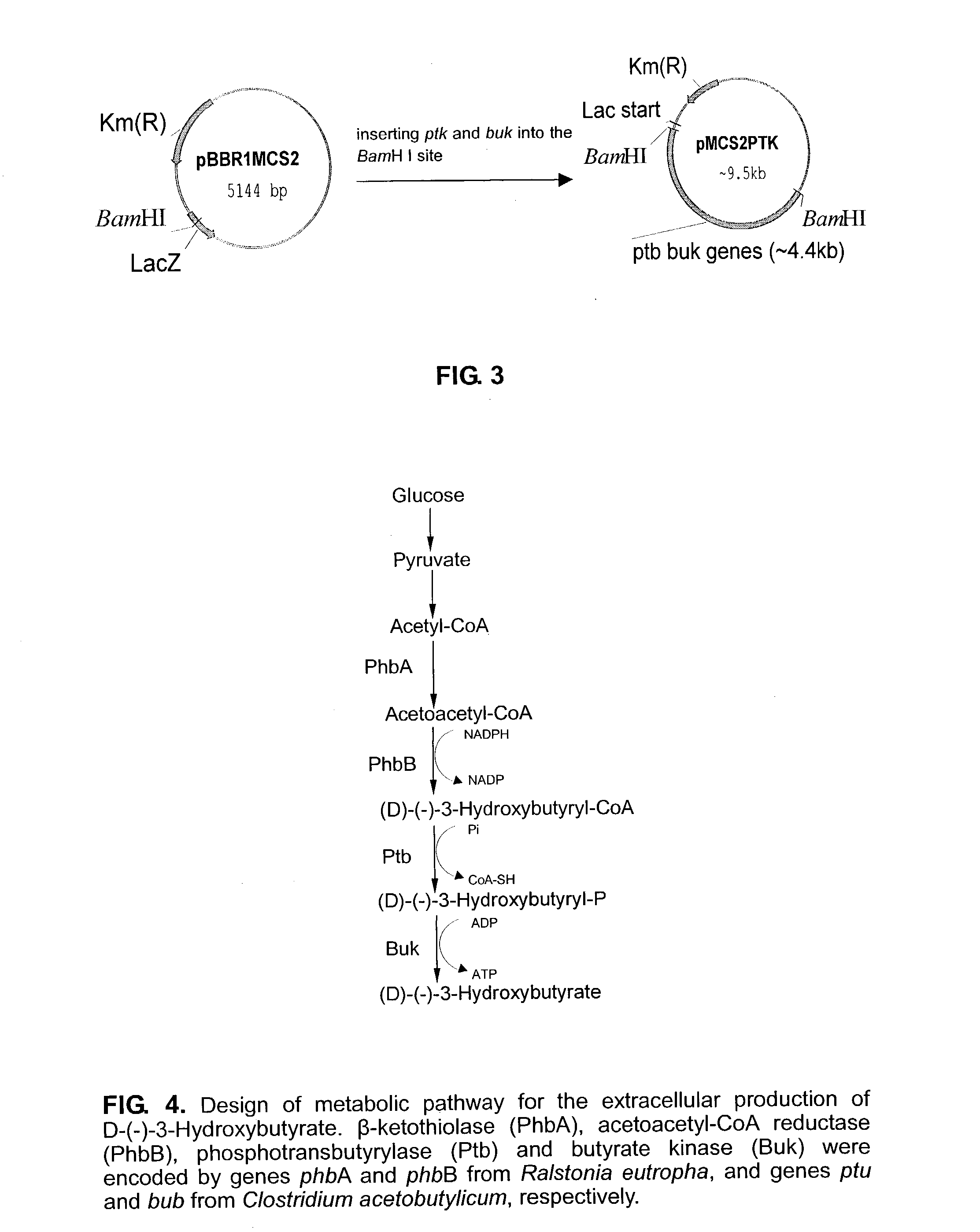
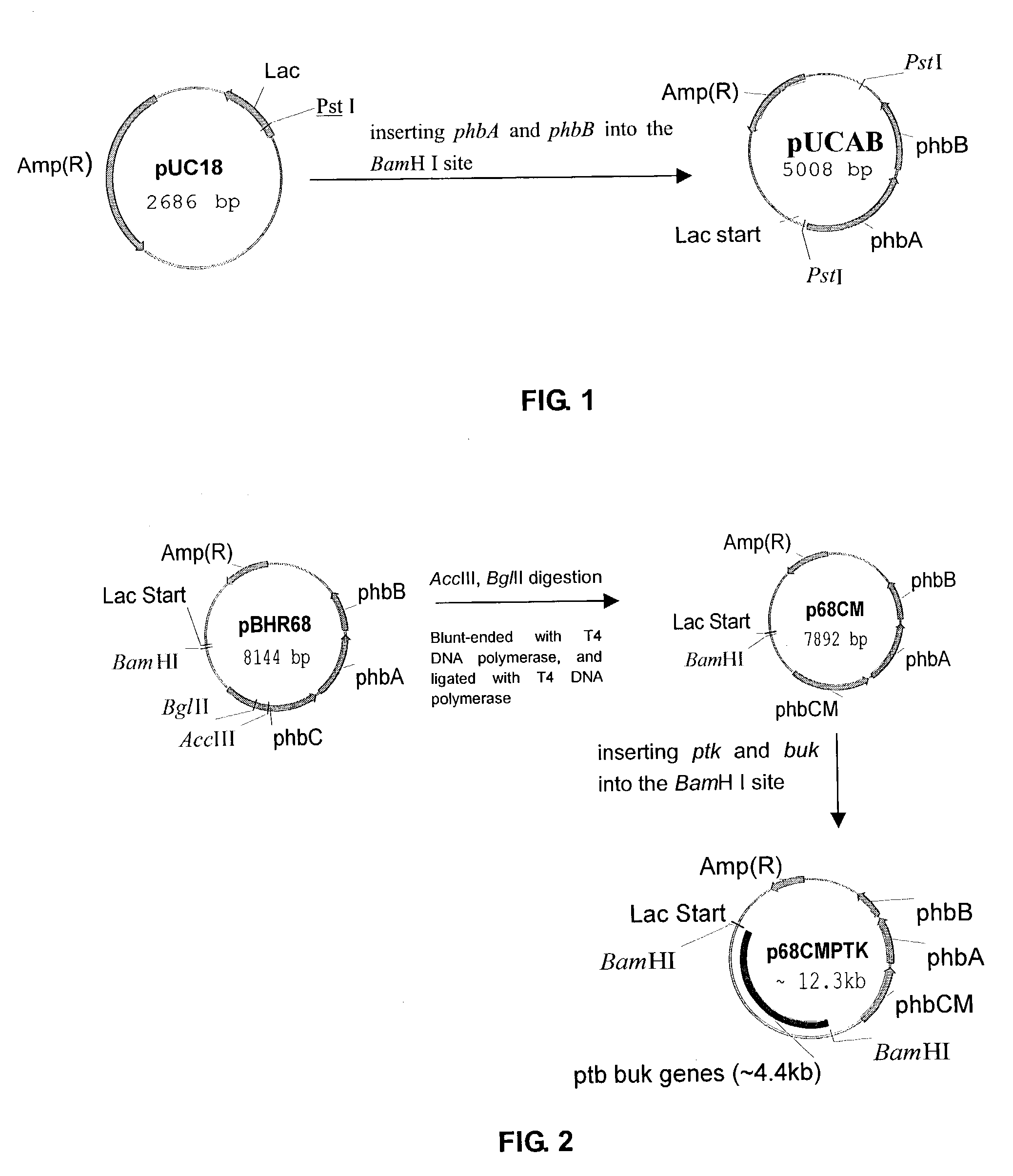
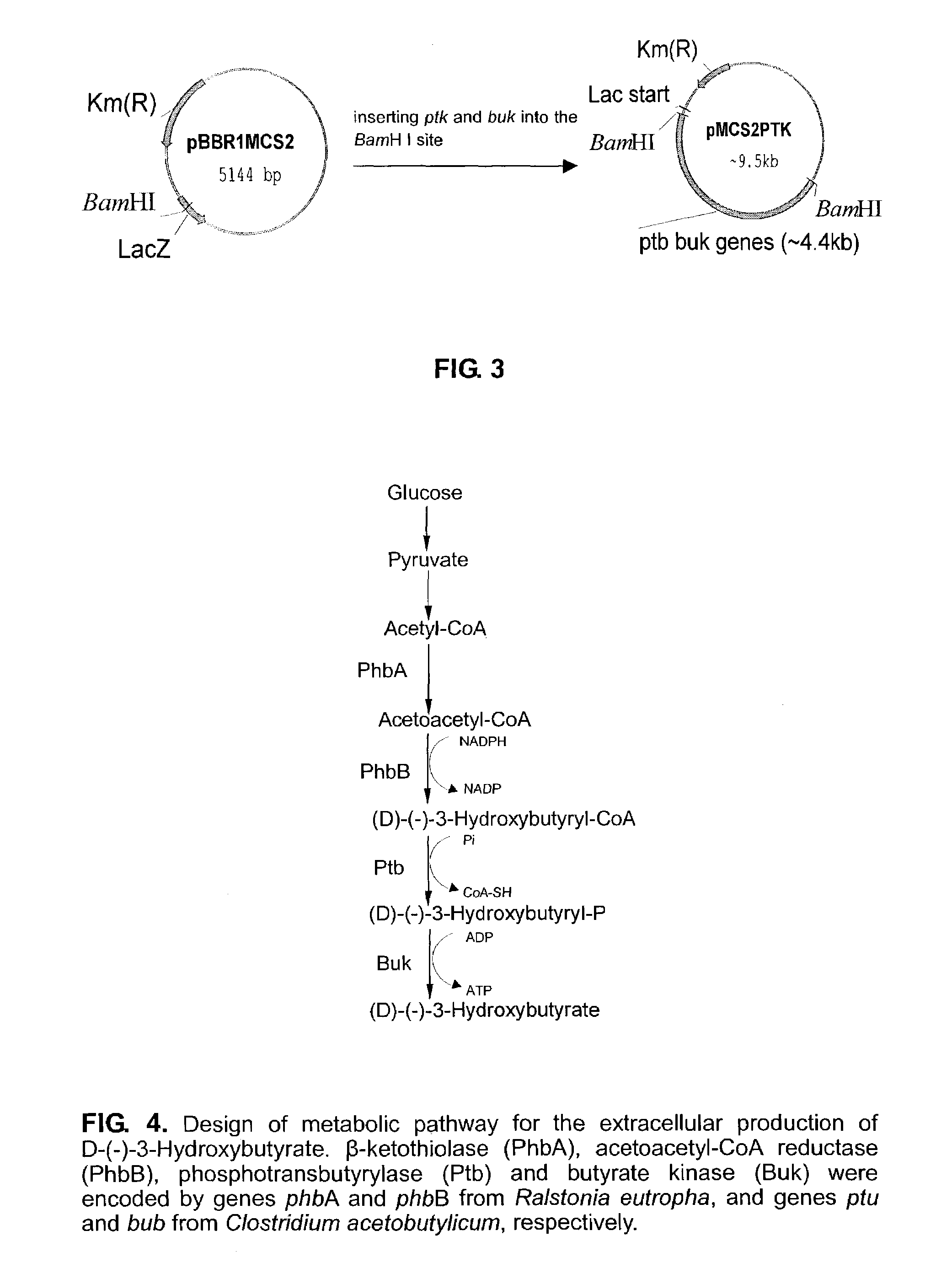
This invention relates to a method for the production of D-(-)-3-hydroxybutyric acid, comprising the step of culturing a recombinant strain containing genes phbA, phbB, ptb and buk by fermentation. Preferably, the recombinant strain is a strain of E. coli. The method of the invention is simple, avoiding the technique of degrading polymer to produce D-(-)-3-hydroxybutyric acid. The present method also provides improved efficiency, lowers the complicated requirement for facilities as used in traditional chemical synthesis, simplifies the complicated technique flow, and omits the complicated chiral separation step. Therefore, the present method greatly reduces the costs associated with D-(-)-3-hydroxybutyric acid production. Also, with this invention, the problems such as environmental pollution of chemical synthesis and chiral separation are overcome.
Owner:CHEN GUOQIANG +2
Method for producing optically active 2-(n-substituted aminomethyl)-3-hydroxybutyric acid ester
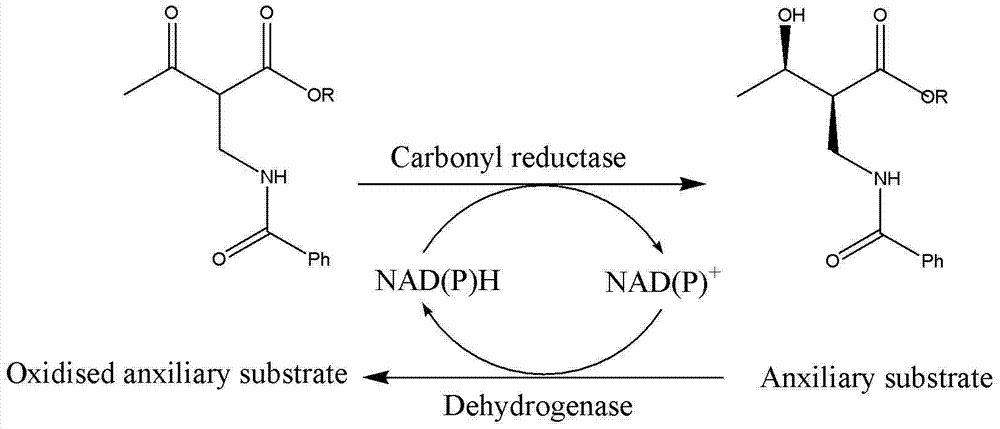
The present invention relates to a method for producing optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid esters wherein a 2-(N-substituted aminomethyl)-3-oxobutyric acid ester is treated with an enzyme source capable of stereoselectively reducing said ester to the corresponding optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid ester having the (2S,3R) configuration. The present invention provides an efficient method for industrially producing optically active 2-(N-substituted aminomethyl)-3-hydroxybutyric acid esters, in particular such compounds having the (2S,3R) configuration, which are useful as intermediates for the production of medicinal compounds, among others.
Owner:KANEKA CORP
Synthetic method of oxiracetam
ActiveCN104230777AEasy to separate and purifyRaw materials are cheap and easy to getOrganic chemistry methodsAlcoholEsterification reaction
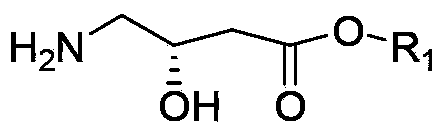
The invention discloses a synthetic method of (S)-oxiracetam. The synthetic method comprises the following steps: (1) by adopting S-4-amino-3-hydroxybutyric acid as a starting material, performing esterification reaction with alcohol to obtain an intermediate I; (2) performing condensation reaction between the intermediate I and halogenated acetic ester to obtain an intermediate II; (3) performing ring closing reaction on the intermediate II to obtain an intermediate III; and (4) performing ammonolysis reaction on the intermediate III to obtain a target product namely (S)-oxiracetam. By adopting a synthetic route of oxiracetam disclosed by the invention, at least more than 20% of an (S)-oxiracetam product with relatively ideal yield can be obtained, and a new oxiracetam synthetic route can be created.
Owner:CHONGQING RUNZE PHARM CO LTD +1
Method for the production of D-(-)-3-hydroxybutyric acid by recombinant Escherichia coli
InactiveUS7262037B2Simple technologyImprove efficiencyBacteriaSugar derivativesEscherichia coliHydroxybutyric acid
This invention relates to a method for the production of D-(−)-3-hydroxybutyric acid, comprising the step of culturing a recombinant strain containing genes phbA, phbB, ptb and buk by fermentation. Preferably, the recombinant strain is a strain of E. coli. The method of the invention is simple, avoiding the technique of degrading polymer to produce D-(−)-3-hydroxybutyric acid. The present method also provides improved efficiency, lowers the complicated requirement for facilities as used in traditional chemical synthesis, simplifies the complicated technique flow, and omits the complicated chiral separation step. Therefore, the present method greatly reduces the costs associated with D-(−)-3-hydroxybutyric acid production. Also, with this invention, the problems such as environmental pollution of chemical synthesis and chiral separation are overcome.
Owner:CHEN GUOQIANG +2
Resource-renewable and biodegradable conductive fiber and preparation method thereof
ActiveCN102936761ARealize conductive functionLower percolation thresholdElectroconductive/antistatic filament manufactureConjugated synthetic polymer artificial filamentsFiber3-Hydroxypentanoic acid
The invention discloses resource-renewable and biodegradable conductive fiber and a preparation method thereof. The fiber consists of the following raw materials in parts by weight: 30-70 parts of polylactic acid (PLA), 30-70 parts of poly(3-hydroxybutyric acid-co-3-hydroxyvalerate (PHBV) and 0.05-8 parts of conductive filler. The preparation method of the conductive fiber comprises the following steps of: (1) proportionally pre-mixing PHBV (if PLA is not less than 50 parts) or PLA (if PLA is less than 50 parts) and the conductive filler, and performing melt blending and granulation to obtain the conductive master batch of the PHBV or PLA; (2) proportionally pre-mixing the PLA or PHBV and the conductive master batch of the PHBV or PLA, and performing melt blending and granulation to obtain composite conductive mater batches; and (3) spinning and drafting the composite conductive master batches one by one to obtain the conductive fiber. The conductive fiber disclosed by the invention can be used as the material for electrodes, static resistance, low-temperature heating, electromagnetic shielding, thermal sensitivity, gas sensitivity and the like.
Owner:JIANGNAN UNIV
Enzymatic processes for the production of 4-substituted 3-hydroxybutyric acid derivatives
The present invention provides methods and compositions for preparing 4-substituted 3-hydroxybutyric acid derivatives by halohydrin dehalogenase-catalyzed conversion of 4-halo-3-hydroxybutyric acid derivatives. The present invention further provides methods and compositions for preparing 4-halo-3-hydroxybutyric acid derivatives by ketoreductase-catalyzed conversion of 4-halo-3-ketobutyric acid derivatives The present invention also provides methods and compositions for preparing vicinal cyano, hydroxyl substituted carboxylic acid esters.
Owner:CODEXIS INC
Blood serum metabolism biological marker for lung cancer patients
InactiveCN101806805AAnalysis using nuclear magnetic resonanceBiological testingMetaboliteSuspected lung cancer
The invention relates to a blood serum metabolism biological marker for lung cancer patients. The blood serum of lung cancer patients and controls is respectively mixed with D2O to form sample solution. A 1H NMR spectrum is collected. The hydrogen spectrum data of the sample is imported into a SIMCA-P software for pattern identification analysis. Compared with the blood serum of the ordinary people, the blood serum of the lung cancer patients have a plurality of metabolites the level of which is higher like lactic acid, dextrose, alanine, valine, leucine, isoleucine, glycine, glutamine, proline, acetacetate, glycoprotein, choline and 3-hydroxybutyric acid. The dextrose and the lactic acid are often influenced by personal factors and are not fit to be the marker. Other metabolites are more stable and almost not influenced by outside environment and can serve as the clinical diagnosis marker. Thereby, a model is established to discriminate the blood serum of the lung cancer patients from the blood serum of the ordinary people and divide into two types. According to the model, the blood serum of the suspected lung cancer case is put in the model to analyze. If the blood serum map of the patient belongs to one of the two types, the patient can be diagnosed as lung cancer or normal.
Owner:HUAZHONG NORMAL UNIV
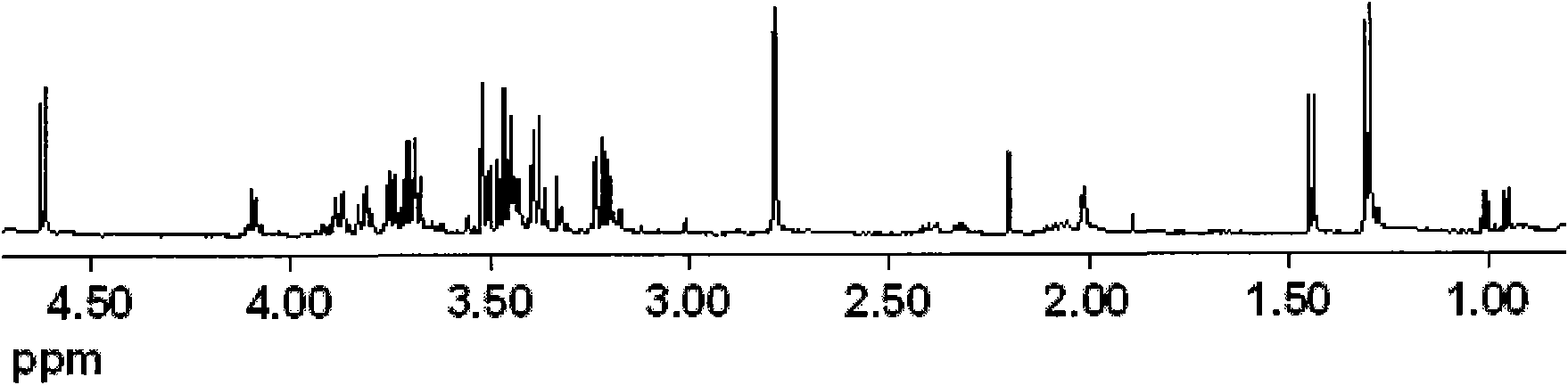
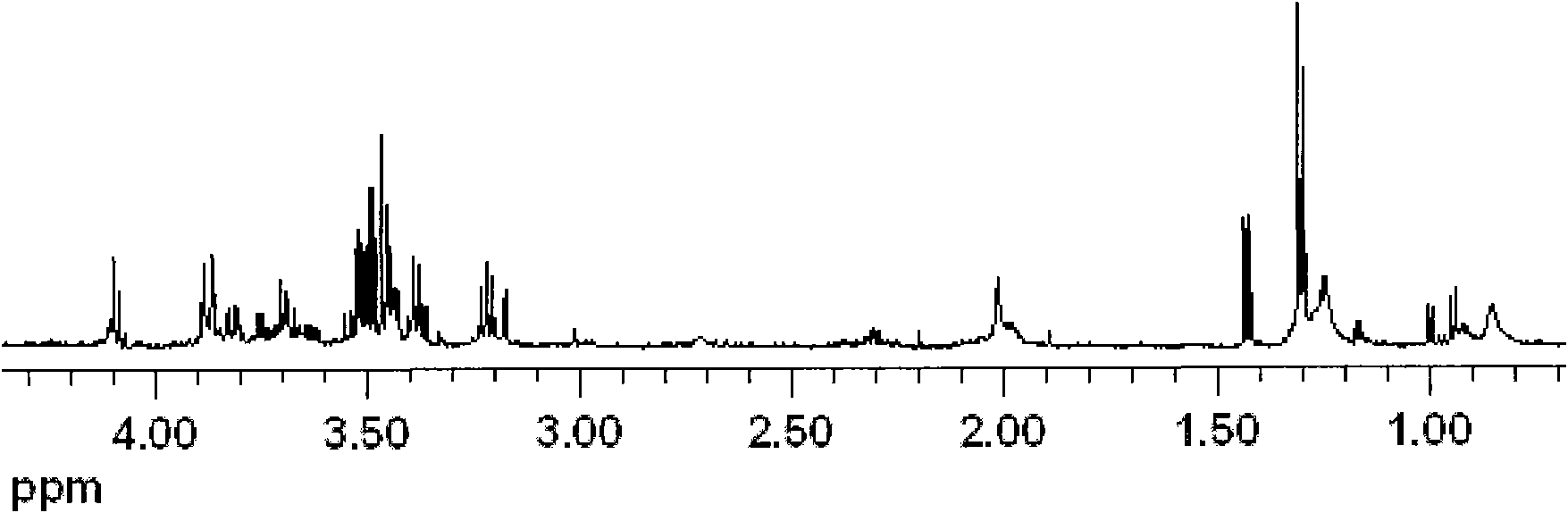
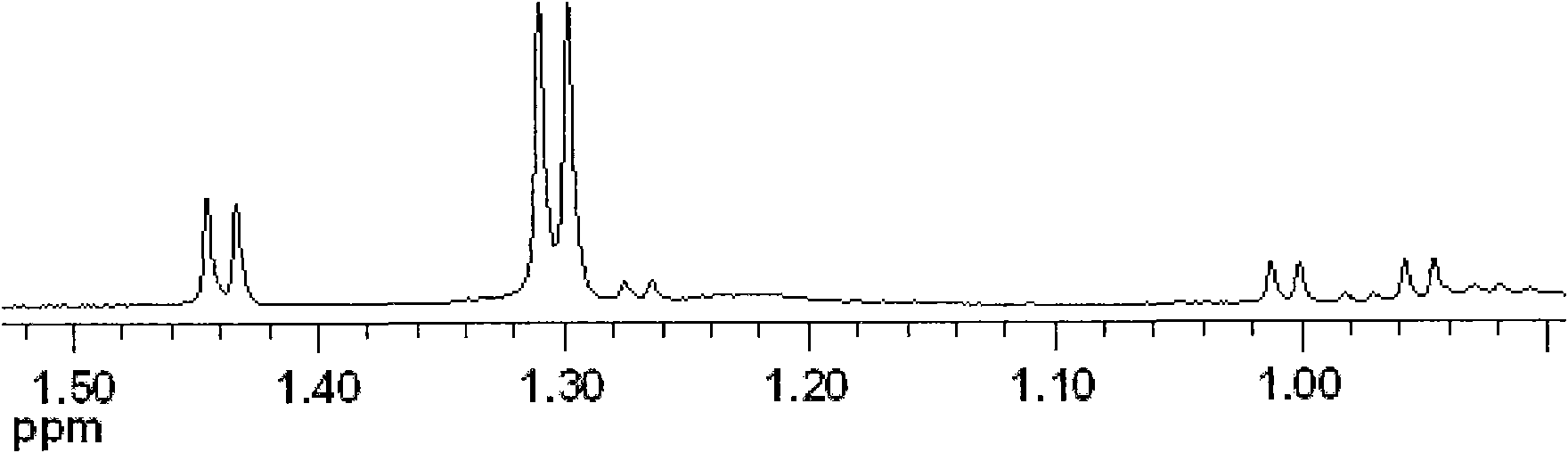
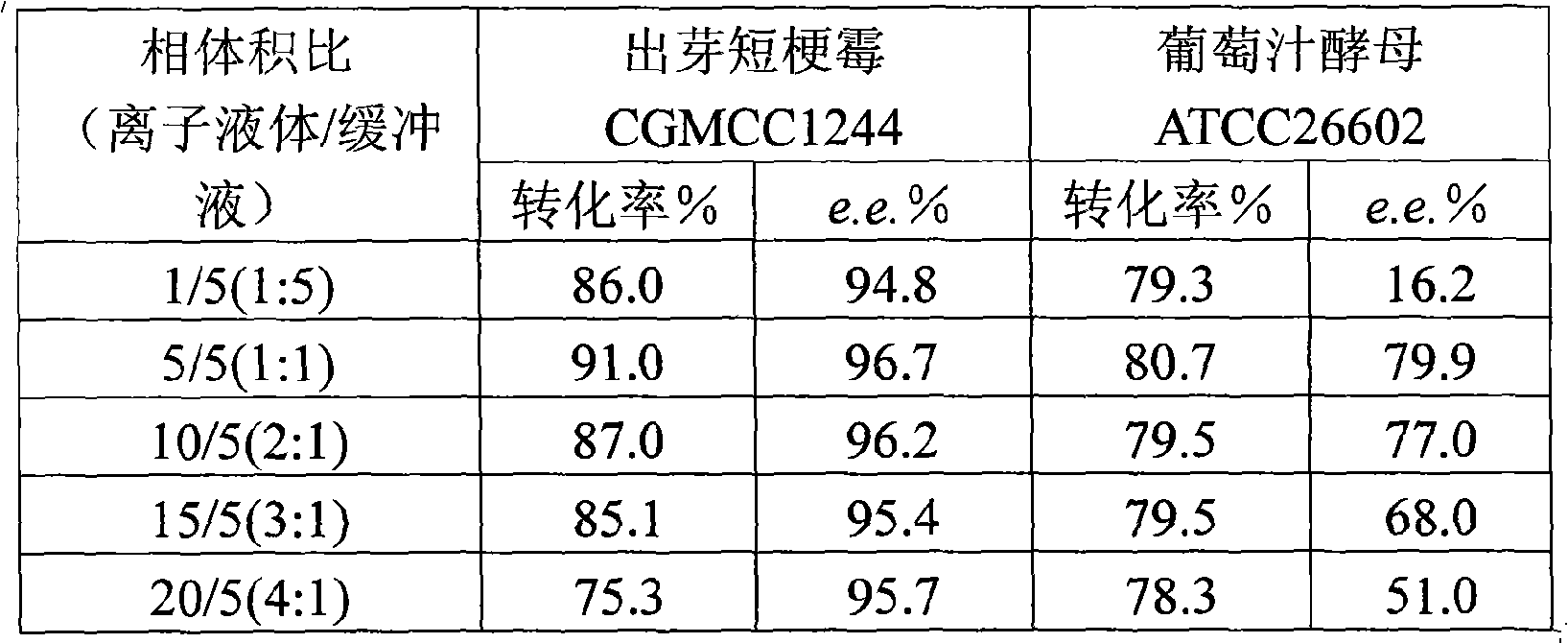
Method for biological catalysis of unsymmetrical reduction carbon based compound in water/ion liquid diphasic system
InactiveCN101319236AEasy to separateEasy to recycleMicroorganism based processesFermentationHydroxybutyric acidBiocompatibility Testing
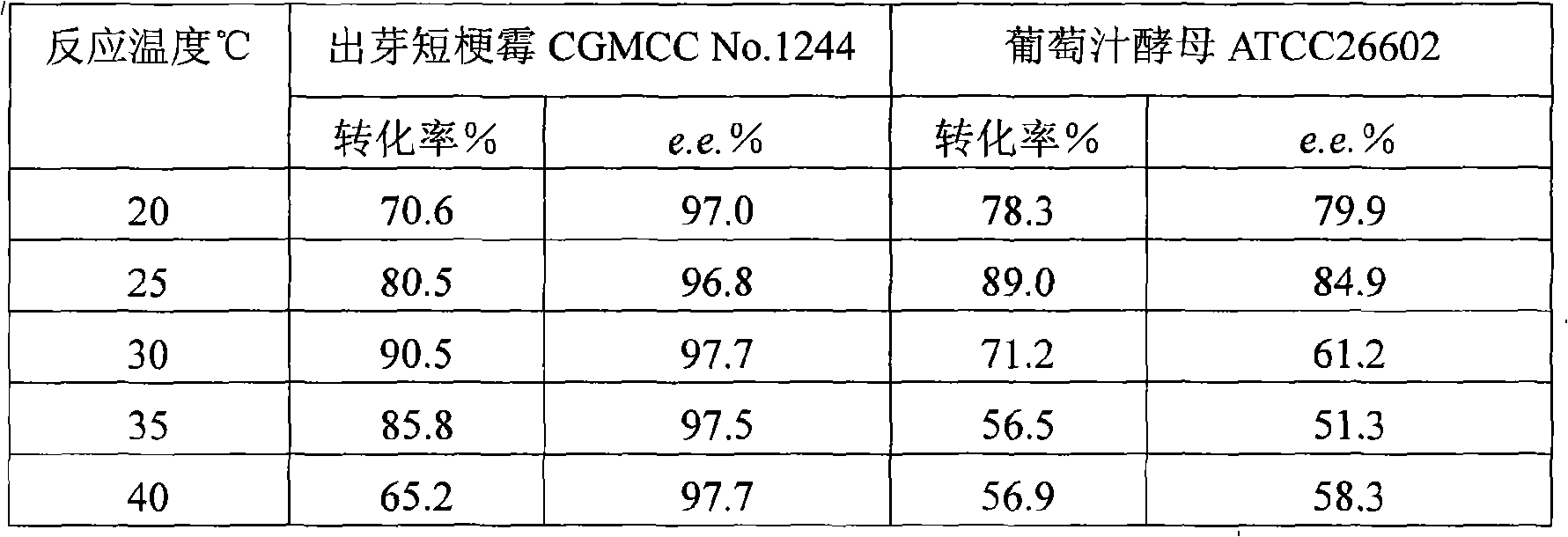
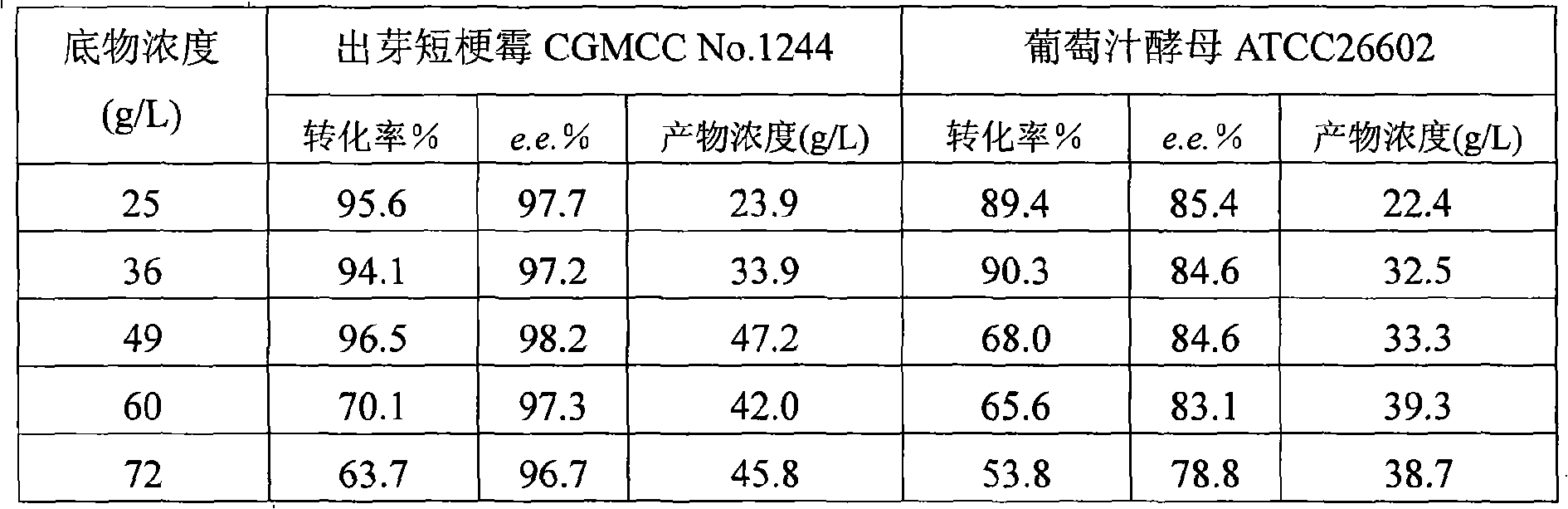
The invention discloses a method for asymmetrically reducing a carbonyl compound by biocatalysis in a water / ion liquid biphasic system, which belongs to the biochemical engineering technical field. The method takes a selective strain producing carbonyl reductase as a starting strain, and prochiral ketone as a substrate to perform reducing preparation of corresponding chiral alcohol in the water / ion liquid biphasic system; the method comprises the following steps that: Aureobasidium pullulans CGMCC No.1244 is used to catalyze 4-chloro-acetoacetic acid ethyl ester to perform the asymmetrical reduction preparation of (S)-4-chloro-3-hydroxybutyric acid ethyl ester, and Saccharoinyces uvarum ATCC 26602 is used to catalyze 4,4,4-trifluoroacetoacetate to perform the asymmetrical reduction preparation of (R)-4, 4, 4-trilfluoro-3-hydroxybutyrate. The method shows the advantages of no toxicity, no smell, difficult volatilization, good biocompatibility, no environmental pollution, simple product separation, easy recovery, repeated use and so on, of ion liquid. At the same time, the method improves the transformation rate, the concentration and an enantiomeric excess value of a reaction product, and quickens the process of the reaction.
Owner:JIANGNAN UNIV
Microorganism catalysis prepared (2S,3R)-2-benzoyl aminomethyl-3-hydroxybutyric acid ester and bacterial strain
ActiveCN103045504ASynthetic reaction conditions are mildEasy to operateBacteriaMicroorganism based processesMicroorganismMicrobial transformation
The invention provides Burkholderia gladioli ZIB-12126, which is a new bacterial strain capable of catalyzing asymmetrical reduction of carbonyl, and application of the bacterial strain in preparation of (2S,3R)-2-benzoyl aminomethyl-3-hydroxybutyric acid ester by racemizing 2-benzoyl aminomethyl-3-hydroxybutyric acid ester in a microbial conversion mode. The bacterial strain is preserved in the Chinese Typical Culture Collection Center with an address of Wuhan College, Wuhan, China and the zip code of 430072, the preservation serial number of CCTCC No. M 2012379 on September 255th, 2012. The bacterial strain provided by the invention is used for synthesizing the (2S,3R)-2-benzoyl aminomethyl-3-hydroxybutyric acid ester through biologic conversion, with gentle reaction condition and environmental-friendliness; and more importantly, the product configuration is mainly (2S,3R) configuration, and step of turning the product configuration by using a chemical method is not needed, so that the operation process is simple and has good industrial application prospects.
Owner:ZHEJIANG UNIV OF TECH
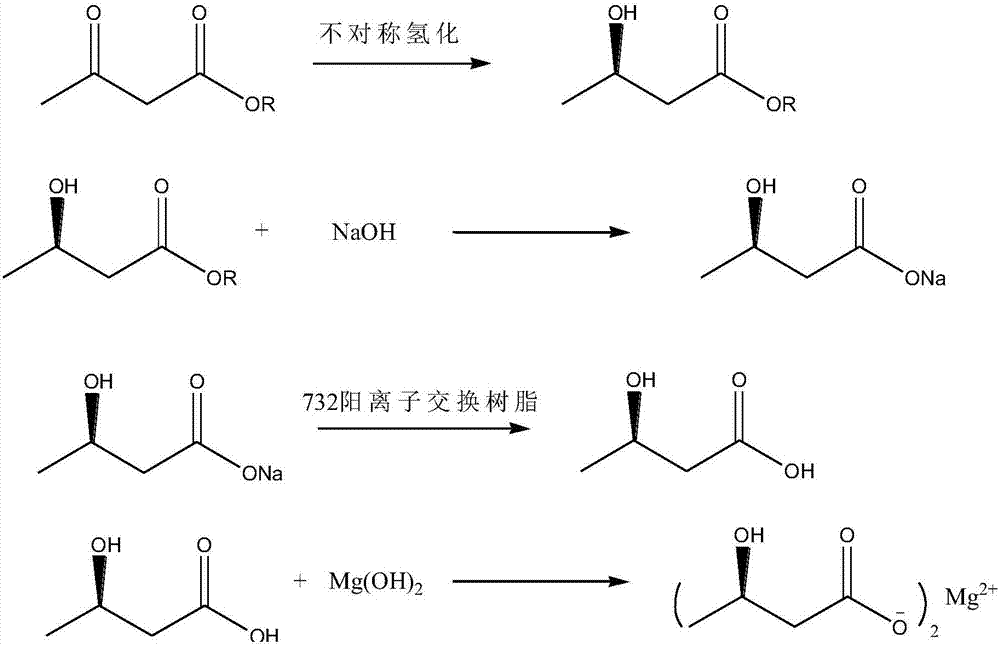
Synthesis process of (R)-3-hydroxybutyric acid and salts thereof
InactiveCN107162893AHigh ee valueReduce pollutionPreparation from carboxylic acid saltsOrganic compound preparationHydrogen pressureMagnesium salt
Owner:洛阳华荣生物技术有限公司
Production method of (R)-3-hydroxybutyric acid
ActiveCN107083406AEnsure food safetyEfficient accumulationBacteriaMicroorganism based processesHydroxybutyric acidMagnesium salt
The invention discloses a method for producing (R)-3-hydroxybutyric acid through one-step fermentation of a microbe, and the method adopts a non-pathogenic microbe and directly converts a carbon source and a nitrogen source into (R)-3-hydroxybutyric acid through fermentation. The produced (R)-3-hydroxybutyric acid can be further prepared into salts such as a sodium salt, a potassium salt, a magnesium salt, a calcium salt and the like to be used as medicinal active components or nutritional supplements, so that the (R)-3-hydroxybutyric acid has extensive industrial application prospects. The microbe used in the method comprises corynebacterium glutamicum constructed through genetic engineering, and the corynebacterium glutamicum is preserved in the China General Microbiological Culture Collection Center, and the preservation number is CGMCC No. 13957.
Owner:ZHEJIANG HUARUI BIOTECHNOLOGY CO LTD
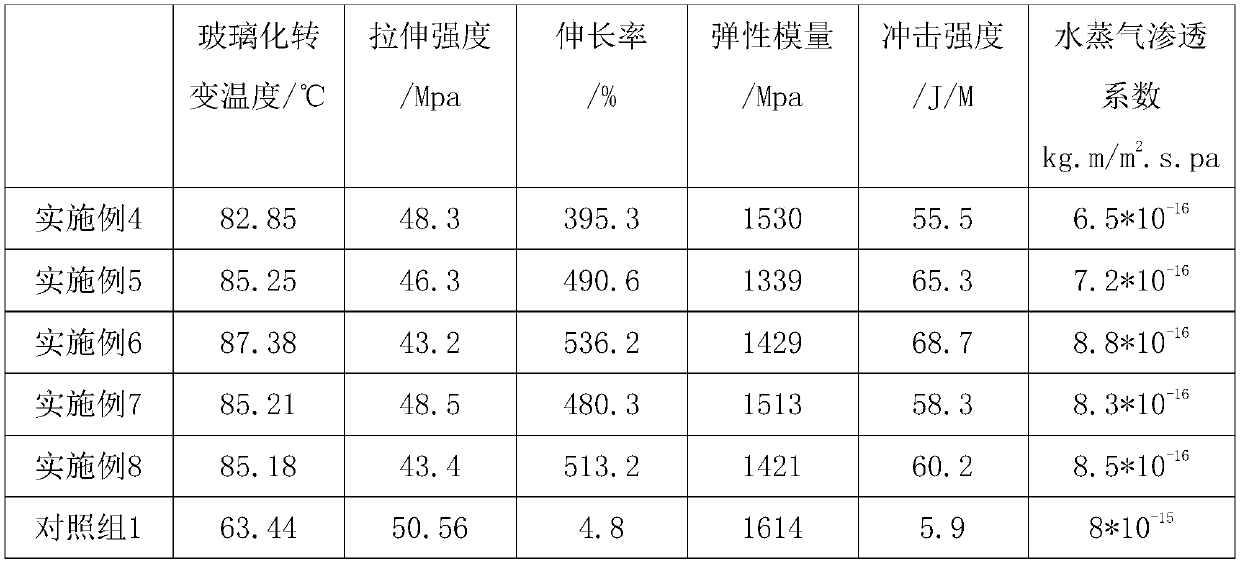
Biodegradable thermal shrinkage film and preparation method thereof
InactiveCN103483789AHigh tensile strengthMeet the basic characteristicsFlat articlesPolymer scienceAntioxidant
The invention discloses a biodegradable thermal shrinkage film. The biodegradable thermal shrinkage film is mainly prepared from the following raw materials in parts by weight: 90 to 100 parts of poly-3-hydroxybutyric acid-4 polyhydroxybutyrate or poly-3 polyhydroxybutyrate, 1 to 5 parts of aid, 2 to 5 parts of lubricating agent, 1 to 3 parts of plasticizer and 2 to 5 parts of antioxidant. The poly-3-hydroxybutyric acid-4 polyhydroxybutyrate or poly-3 polyhydroxybutyrate is a biodegradable macromolecular material and has the advantages of high mechanical strength, thermal formability, biocompatibility and the like, so that the thermal shrinkage film prepared by combining the poly-3-hydroxybutyric acid-4 polyhydroxybutyrate or poly-3 polyhydroxybutyrate with the acid, a toughening agent and thr like has high tensile strength, accords with the basic characteristics of the heat shrinkage film, can be biodegraded completely, and is an environment-friendly biological film.
Owner:太仓市鸿运包装材料有限公司
High-mechanical strength and biodegradable PLA-PHBV composite material, preparation method thereof and thin film
The invention discloses a high-mechanical strength and biodegradable PLA-PHBV composite material. The composite material contains the following components in parts by weight: 40-80 parts of polylacticacid and 5-30 parts of 3-hydroxybutyric acid-co-3-hydroxyvaleric acid copolymer, 2-15 parts of an ethylene-vinyl acetate copolymer grafted glycidyl methacrylate modified toughening agent, 2-8 parts of a solubilizer and 0.2-0.8 part of a nucleating agent. The composite material belongs to the field of high molecular materials and has the advantages that the mechanical property is improved, meanwhile, the glass transition temperature is high, the crystallization degree is low, and the melting temperature is low. The invention further discloses a preparation method of the composite material anda thin film prepared from the composite material. The thin film can present an excellent barrier property.
Owner:GUANGDONG JUHANG INST FOR ADVANCED MATERIALS CO LTD
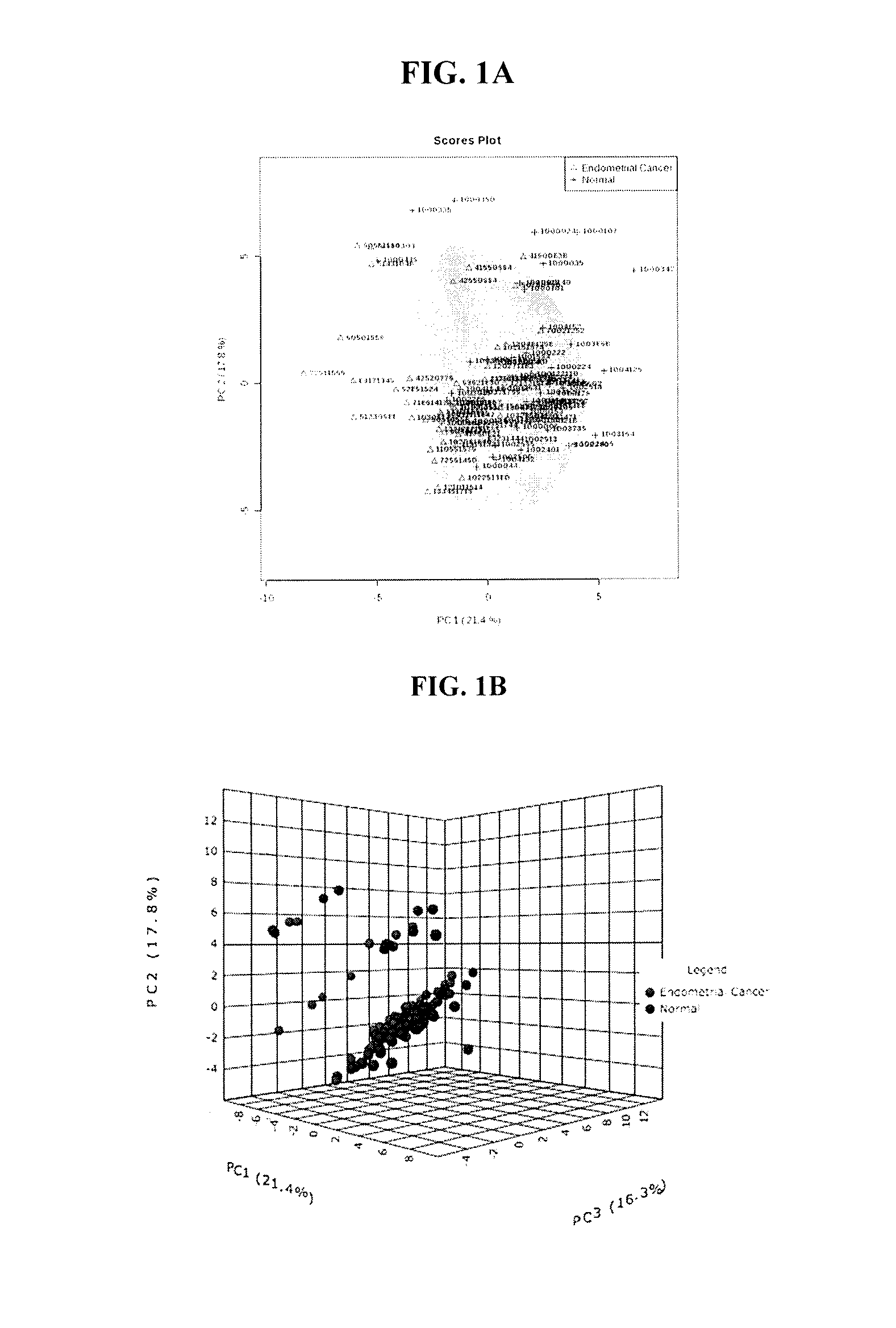
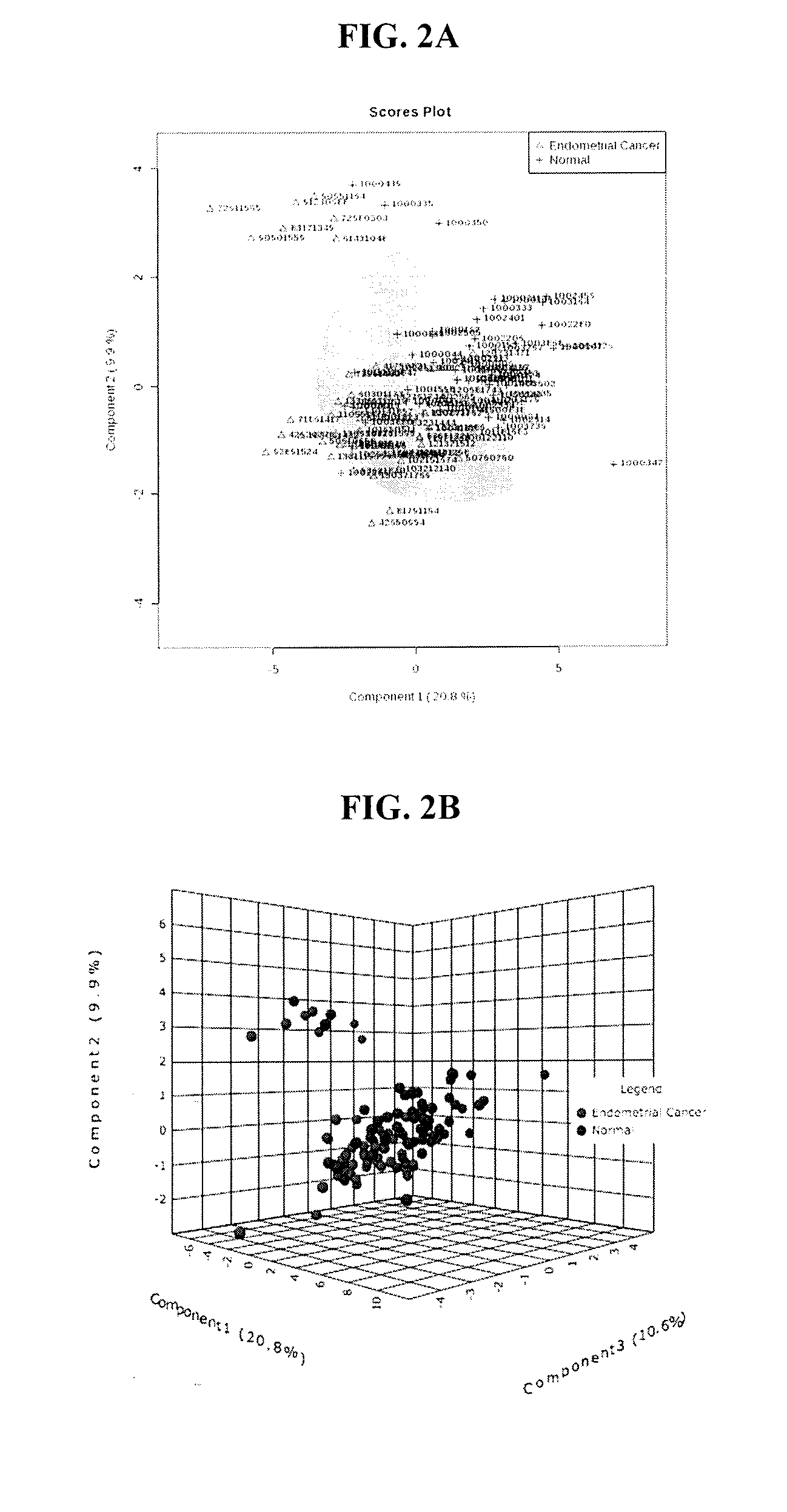
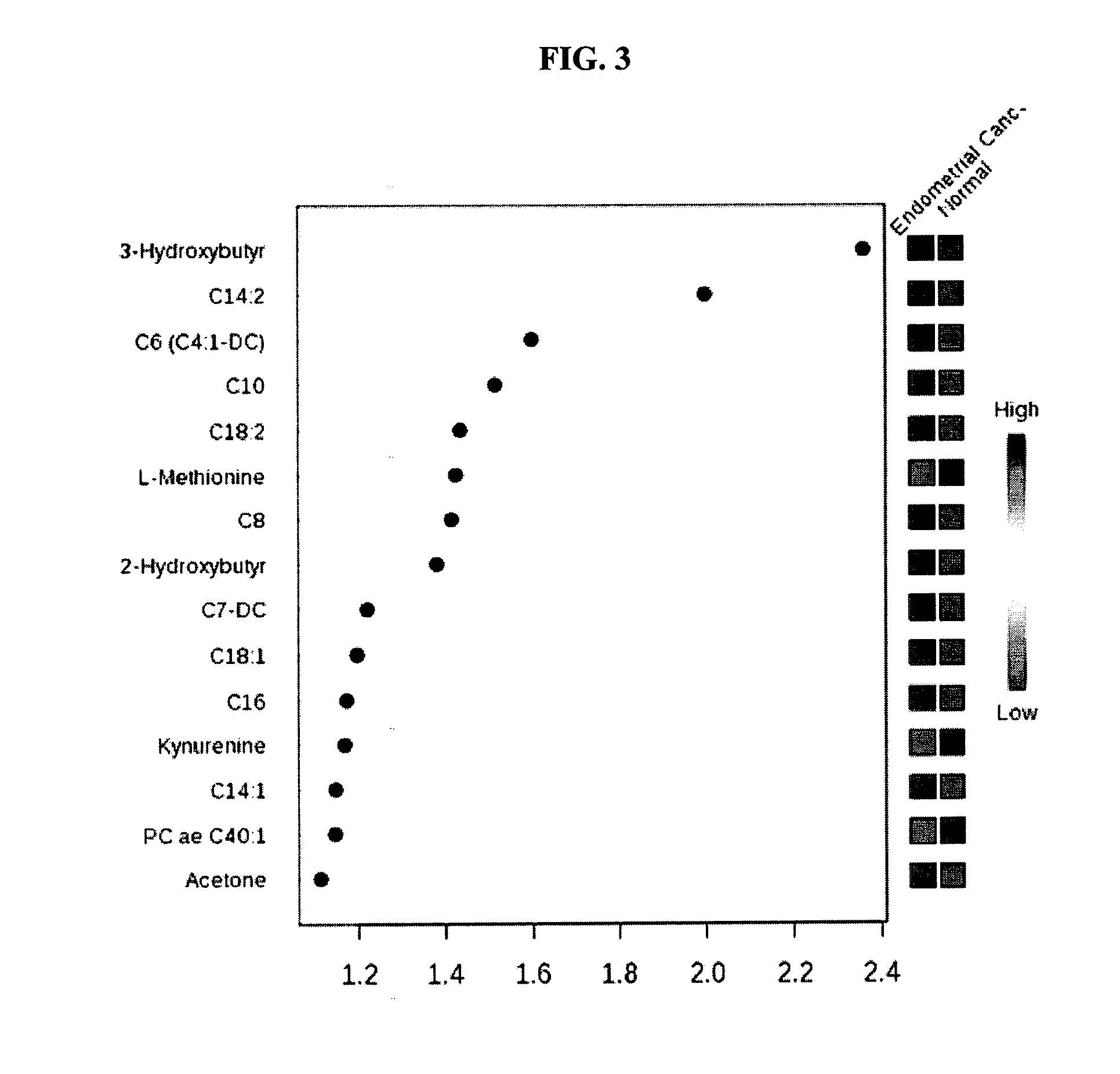
Methods for detecting, diagnosing and treating endometrial cancer
InactiveUS20170003291A1Improve diagnostic accuracyImproved prognosisOrgan movement/changes detectionOmicsMetaboliteOncology
The present invention relates to methods for detecting, diagnosing and / or treating endometrial cancer by detecting in a biological sample from a patient the levels of one or more of the metabolites: C14.2, PC ae C38:1, 3-Hydroxybutyric acid, C18:2, PC ae C40:1, and C6 (C4:1-DC). In some embodiments, the method also includes diagnosing the patient with endometrial cancer when the one or more metabolites in the biological sample is at a different level than a statistically validated threshold for the one or more metabolites, and ultrasound indicates endometrial cancer in the patient. In further embodiments, once endometrial cancer is diagnosed, the patient is treated for the endometrial cancer.
Owner:WILLIAM BEAUMONT HOSPITAL
Organism coupling preparation method of R(-)-4-cyan-3-hydroxybutyric acid ethyl ester
InactiveCN101914582AReduce manufacturing costHigh chemical purityFermentationChemical synthesisEthyl butyrate
The invention relates to an organism coupling preparation method of R(-)-4-cyan-3-hydroxybutyric acid ethyl ester. The method comprises the following steps of: reacting epichlorohydrin as a raw material with sulfuric acid and sodium cyanide in a buffer solution to obtain 4-chlorine-3-hydroxy butyronitrile; then undergoing Pinner reaction by using the 4-chlorine-3-hydroxy butyronitrile and hydrochloric acid ethanol to generate 4-chlorine-3-hydroxybutyric acid ethyl butyrate; and finally acting the 4-chlorine-3-hydroxybutyric acid ethyl butyrate with sodium cyanide and sulfuric acid under the catalysis of halogenohydrin dehalogenation enzyme to obtain R(-)-4-cyan-3-hydroxybutyric acid ethyl ester. By using the lowly-cost and easily-obtained epichlorohydrin as the raw material and the manner of combining chemical synthesis and biological catalysis, the invention greatly reduces the production cost, has temperate reaction condition, simple process and high chemical and optical purity of the product, and has obvious economic and environmental benefits beneficial to industrial production by adopting halogenohydrin dehalogenation enzyme catalysis in industrial production.
Owner:LIANYUNGANG HONGYE CHEM
Diagnosis test paper for diabetic ketosis and other symptoms of relatively high ketone body
InactiveCN101900734AEasy to operateQuick checkMaterial analysis by observing effect on chemical indicatorBiological testingReagent stripBovine serum albumin
The invention relates to diagnosis test paper for diabetic ketosis and other symptoms of relatively high ketone body. The diagnosis test paper comprises the following reagents: (1) first-phase immersion liquid which comprises a stabilizer and a protective agent, bovine serum albumin, oxalic acid, NAD<+>, NADP<+>, diaphorase, 3-hydroxybutyrate dehydrogenase and buffer solution; and (2) second-phase immersion liquid which comprises NBT, PMS and an organic solvent. The diagnosis test paper is prepared by the following steps of: soaking filter paper in the first-phase immersion liquid, sucking redundant immersion liquid and quickly and warmly drying the soaked filter paper at the temperature of between 20 and 50 DEG C; soaking the dried filter paper in the second-phase immersion liquid and drying at the temperature of between 20 and 50 DEG C again to obtain base paper of the diagnosis test paper; and sticking the base paper on a plastic base and cutting into pieces to obtain the diagnosisreagent strips. The diagnosis test paper can be assembled into various detection devices, has the advantages of convenient operation, fast detection, good sensitivity, high accuracy and the like, cansemiquantitatively detect the 3-hydroxybutyric acid content of milk, urine and blood, and has good application prospect.
Owner:SHANGHAI GAOFENG MEDICAL ELECTRICAL EQUIP
Enzymatic method detection kit of D-3-hydroxybutyric acid and preparation method thereof
ActiveCN104730230AImprove protectionImprove stabilityMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementOxalateCacodylic acid
The invention provides an enzymatic method detection kit of D-3-hydroxybutyric acid. The kit comprises a reagent 1 and a reagent 2 according to a ratio of 3: 1. The reagent 1 (with the volume of 3L) comprises 10-200mmol / L of a buffer solution, 50-200U / L of D-3-hydroxybutyric acid dehydrogenase, 1-3g / L of a polyoxypropylene-polyoxyethylene copolymer, 0.1-2g / L of sodium azide and the balance deionized water. The reagent 2 (with the volume of 1L) comprises 10-200mmol / L of oxalate, 0.1-10mmol / L of oxidized coenzyme, 0.1-2g / L of sodium azide and the balance deionized water. The used D-3-hydroxybutyric acid dehydrogenase stabilizing agent can good protect enzyme in the reagent so that the D-3-hydroxybutyric acid kit stability is improved, a cost is greatly reduced and a large clinical application value is obtained.
Owner:SHANGHAI FOSUN PHARMA (GROUP) CO LTD +1
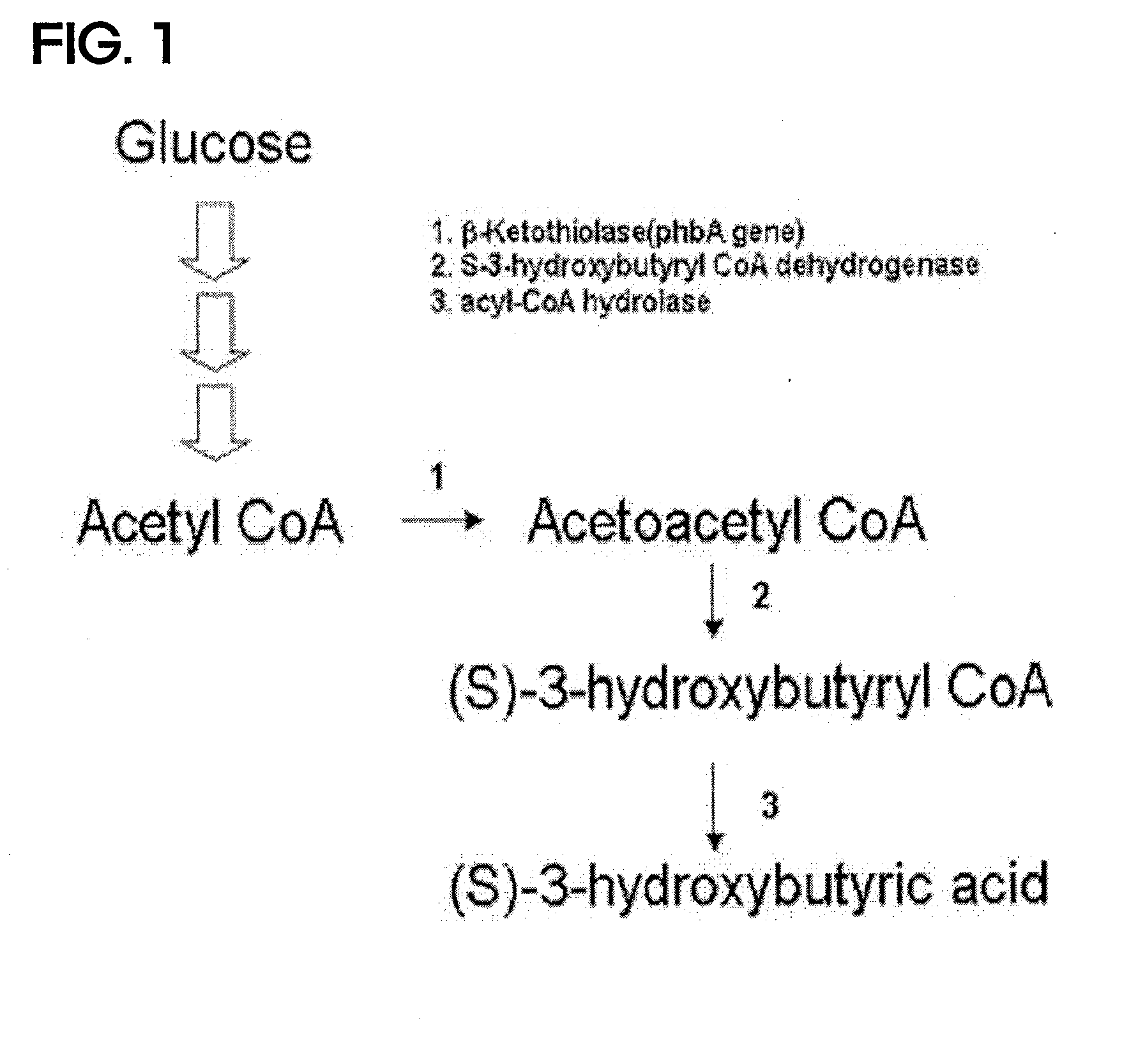
Preparing method for (s)-3hydroxybutyric acid and (s)-3 hydroxybutyrate ester using recombinant microorganism
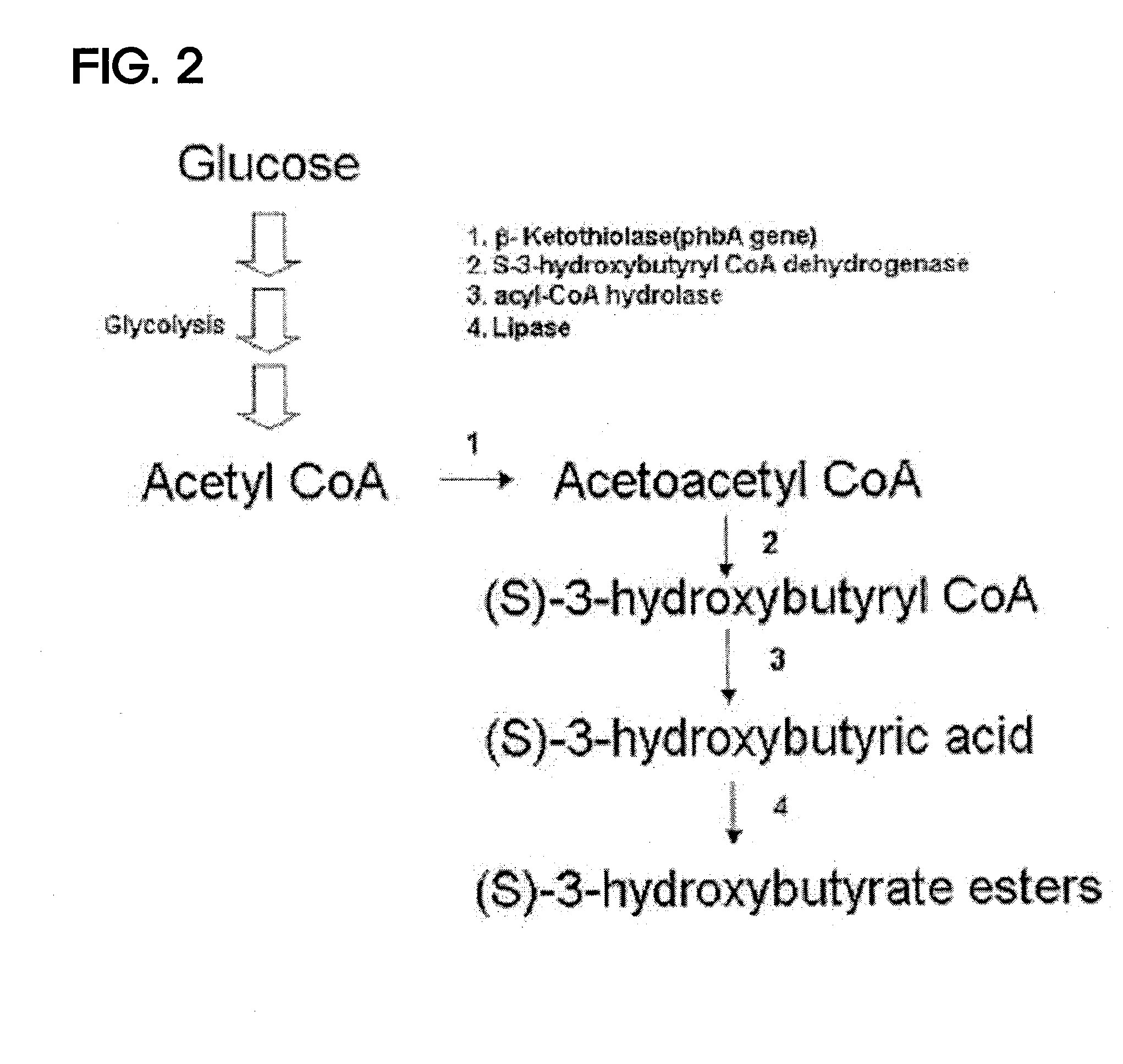
ActiveUS20100209983A1High optical purityFermentationVector-based foreign material introductionBiologyHydroxybutyrates
A method of synthesizing optically-active (S)-3-hydroxybutyric acid and (S)-3-hydroxybutyrate ester using a mutated microorganism is provided. More particularly, a mutated microorganism for preparing (S)-3-hydroxybutyric acid transformed with a gene encoding b ketothiolase, a gene encoding (S)-3-hydroxybutyryl CoA dehydrogenase and a gene encoding acyl CoA hydrolase; a method of preparing (S)-3-hydroxybutyric acid using the mutated microorganism; a mutated microorganism for preparing (S)-3-hydroxybutyrate ester transformed with a gene encoding b ketothiolase, a gene encoding (S)-3-hydroxybutyryl CoA dehydrogenase, a gene encoding acyl CoA hydrolase and a gene encoding lipase; and a method of preparing (S)-3-hydroxybutyrate ester using the mutated microorganism are provided.Accordingly, (S)-3-hydroxybutyric acid with high optical purity may be produced from acetyl CoA produced in glycolysis of a microorganism by a simple process involving the manipulation of a metabolic pathway by a recombinant gene introduced into the microorganism without using a high-cost metal catalyst or a substrate. Further, (S)-3-hydroxybutyrate ester and lactone of (S)-3-hydroxybutyrate ester may be simply produced from (S)-3-hydroxybutyric acid produced by the above method using lipase.
Owner:LG CHEM LTD
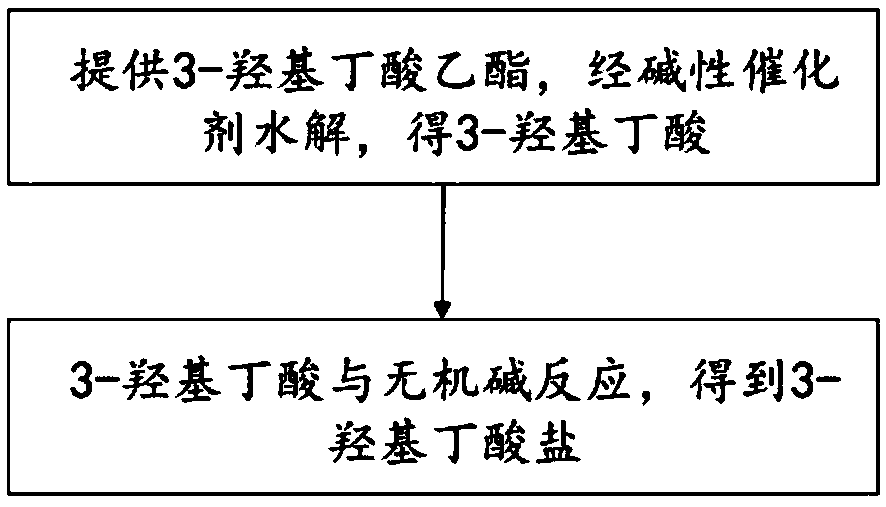
Method for preparing 3- hydroxybutyrate
InactiveCN109369372AReduce lossesThorough responseOrganic compound preparationPreparation from carboxylic acid esters/lactonesAnhydrous ethanolOrganic solvent
The invention discloses a method for preparing 3-hydroxybutyrate. The method comprises the steps that (1) 3-ethyl hydroxybutyrate or 3-methyl hydroxybutyrate is provided and is hydrolyzed through a base catalyst to obtain 3-hydroxybutyric acid; and (2) the 3-hydroxybutyric acid reacts with an inorganic base to obtain the 3- hydroxybutyrate. Through the method, an aquatic salt forming mode is adopted, reacting is more complete, the reaction time is saved, energy consumption and material losses are lowered, the product yield is improved, and the production cost is saved. The concentration process in preparation of 3-hydroxybutyrate crude products is omitted, the series of processes of refining concentration of anhydrous ethanol, adding of acetone for crystallization, filtering, washing, drying and the like in preparing of 3-hydroxybutyrate finished products are omitted, an organic solvent, namely, acetone is omitted, material losses and energy consumption for the corresponding processesare reduced, and the production cost of the 3-hydroxybutyrate is greatly lowered. The heating process in roughing and refining of the 3-hydroxybutyrate is reduced, the problem that the 3-hydroxybutyrate finished products are easily affected with damp is also solved through the aquatic salt forming mode, and the quality of the 3-hydroxybutyrate is guaranteed.
Owner:SHANGHAI SHINE HIGH INT TRADE CO LTD
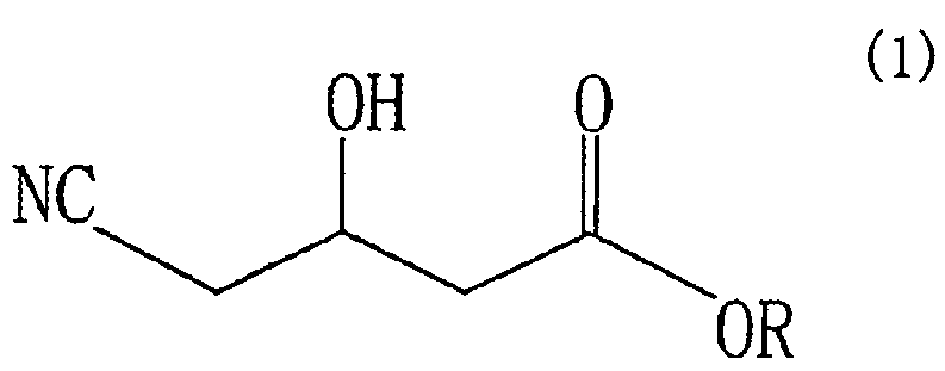
A process for preparing (R)-4-cyano-3-hydroxybutyric acid ester
InactiveCN1337940ACarboxylic acid nitrile preparationOrganic compound preparationBenzyl groupMedicinal chemistry
The present invention relates to a process for preparing (R)-4-cyano-3-hydroxybutyric acid ester products and more particularly, to a process for preparing optically pure (R)-4-cyano-3-hydroxybutyric acid ester products expressed by formula (1) in high yield by performing cyanation and sequential esterification of (S)-3,4-epoxybutyric acid salt as a starting material. In said formula, R represents linear or branched alkyl group with 1-4 carbon atoms or benzyl group. (R)-4-cyano-3-hydroxybutyric acid ester with more than 90% of yield and 99.8% of optical purity.
Owner:LOTTE FINE CHEM CO LTD
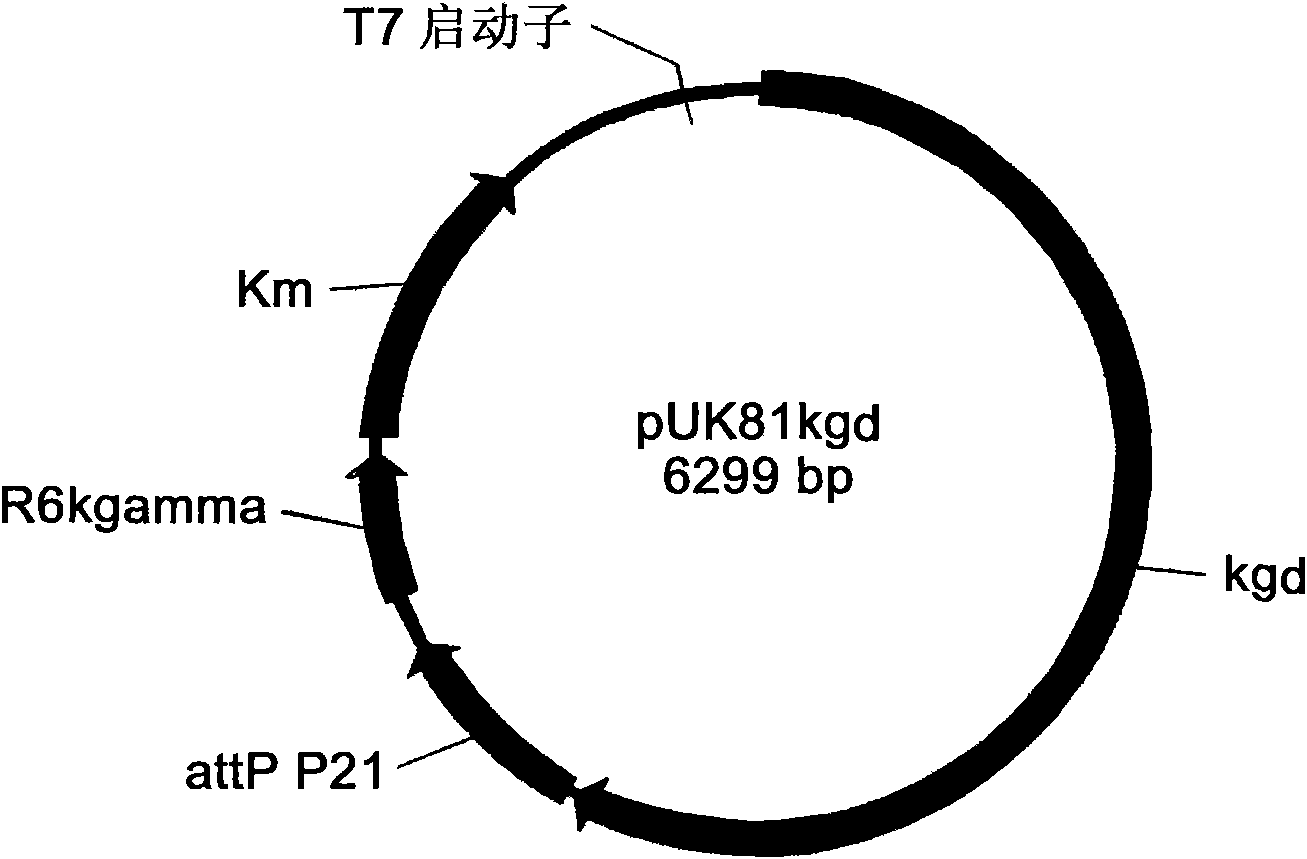
Engineering bacterium containing 2-oxoglutarate decarboxylase gene kgd and applications thereof
InactiveCN102382789AReduce manufacturing costBacteriaMicroorganism based processesCoenzyme A biosynthesis2-Oxoglutarate Dehydrogenase
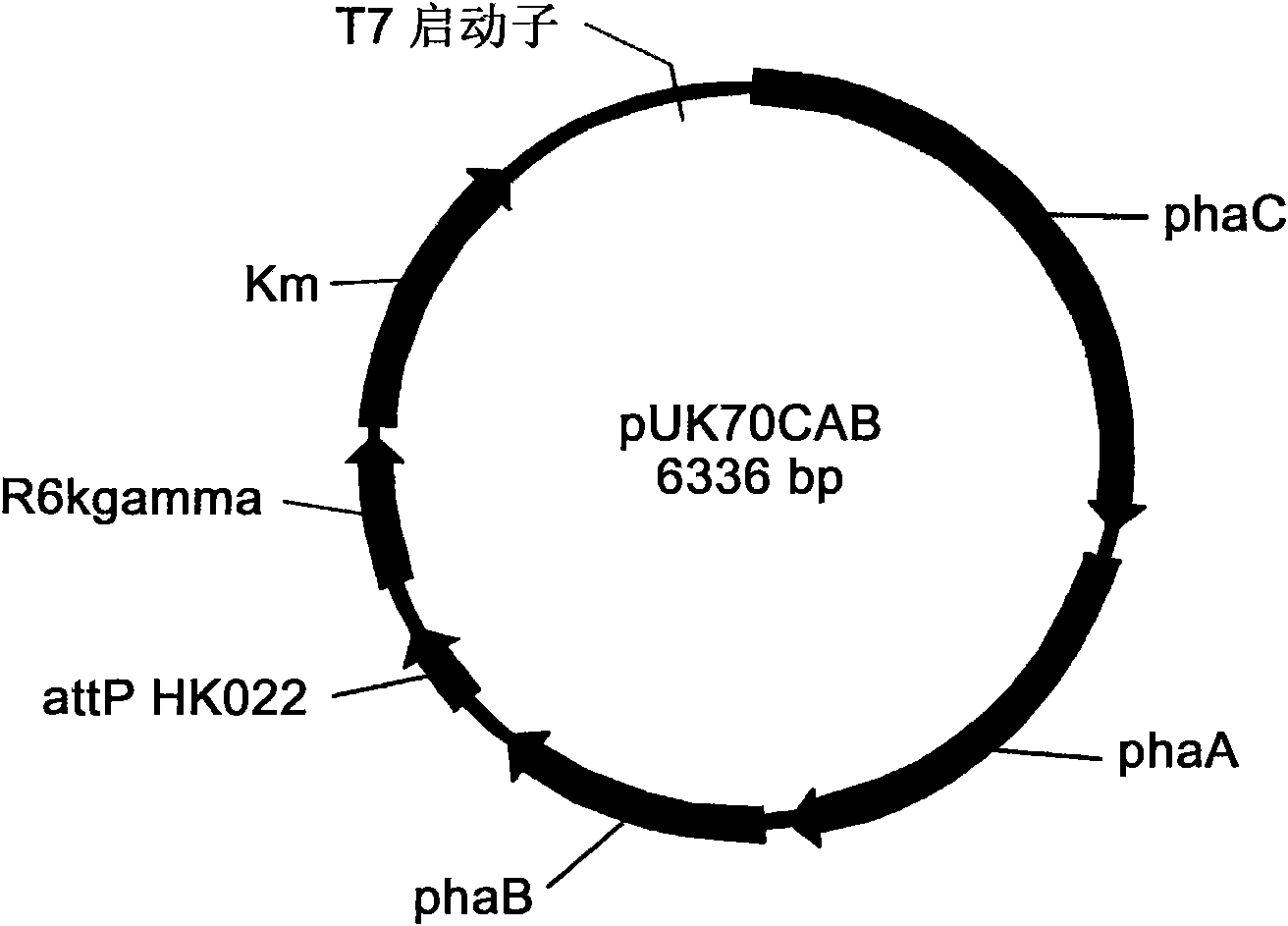
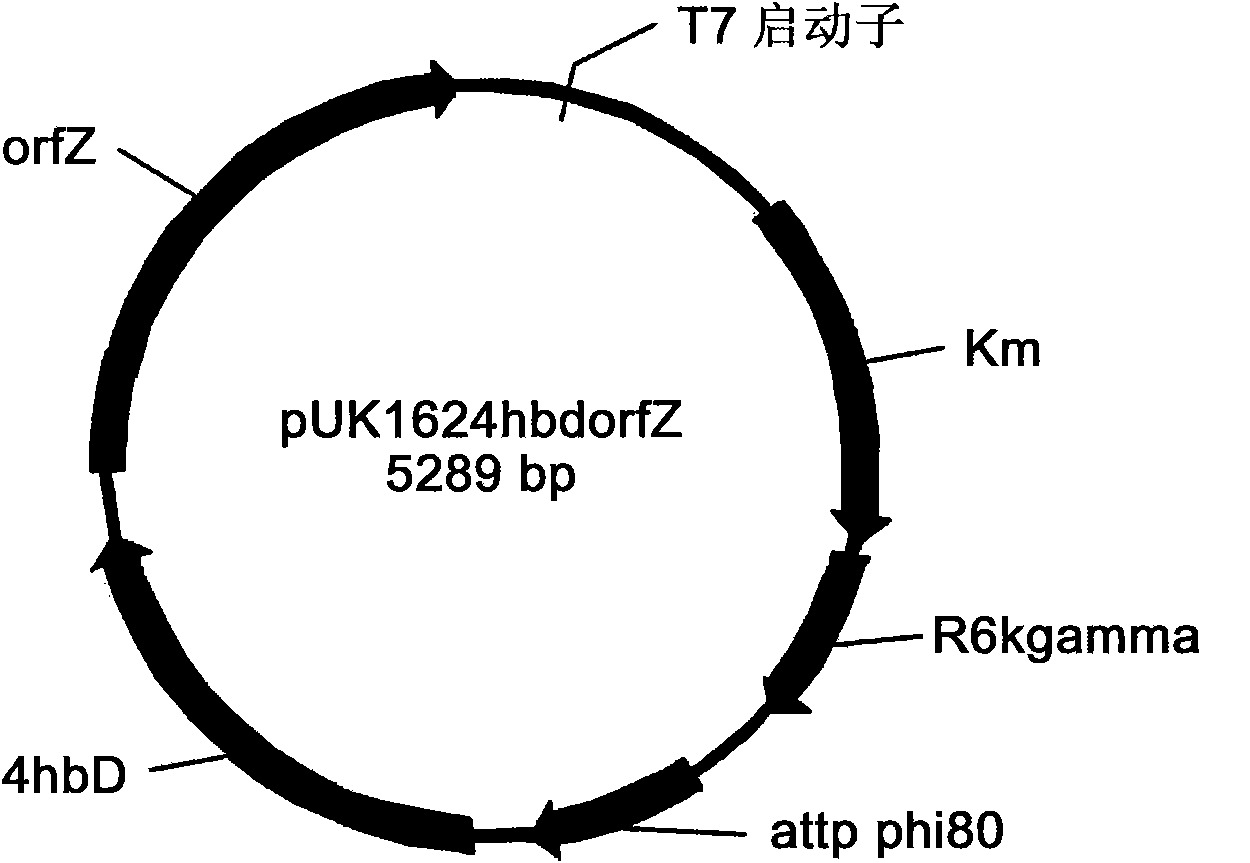
The invention belongs to the technical field of genetic engineering and fermentation, and particularly discloses an engineering bacterium for producing 3-hydroxybutyric acid and 4-hydroxybutyric acid copolyester (P3HB4HB) by utilizing a sugar carbon source. Exogenous genes needed for combining the P2HB4HB are recombined and integrated on the genome of the engineering bacterium and comprise poly-3-hydroxybutyrate synthetic gene phaCAB and 4-hydroxybutyryl coenzyme A which is transferase gene orfZ, 4-hydroxybutyric acid dehydrogenase gene 4hbD and 2-oxoglutarate dehydrogenase gene kgd. By utilization of the engineering bacterium, the P3HB4HB can be produced by using the sugar carbon source with relatively cheap price, the production cost is effectively reduced, and the large-scale industrial production and the commercial application and development are pushed.
Owner:TIANJIN GREENBIO MATERIAL CO LTD
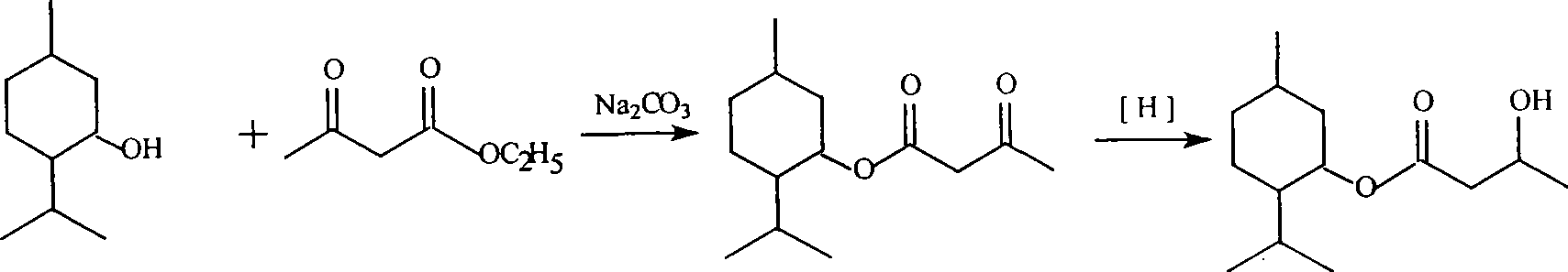
Method for synthesizing cooling agent L-menthyl 3-hydroxybutyrate
InactiveCN101423474ARaw materials are easy to getMild conditionsPreparation by ester-hydroxy reactionReaction temperatureSolvent
The invention discloses a synthetic method for 3-hydroxybutyric acid L-mint ester. The synthetic method comprises two steps: a first step, namely, a synthetic method for acetoacetic acid L-mint ester, which is characterized in that acetoacetic ester or methyl acetoacetate and L-mint camphor are subjected to ester exchange reaction under the action of a basic catalyst to prepare the acetoacetic acid L-mint ester, wherein the reaction time is 4 to 5 hours, the catalyst used in the reaction is sodium carbonate or potassium carbonate or calcium carbonate, and solvent used in the reaction is methyl benzene or benzene or cyclohexane; and a second step, namely, the acetoacetic acid L-mint ester is reduced to obtain the 3-hydroxybutyric acid L-mint ester, wherein the reaction temperature is between 10 and 20 DEG C, the solvent used in the reaction is methanol or ethanol, and a reducer in the reaction is potassium borohydride or sodium borohydride. The synthetic method has the advantages of easily obtained raw materials, mild conditions, simple and convenient operation, and high yield.
Owner:上海香料研究所
Polyhydroxyalkanoate fibers, preparing method and application thereof
ActiveCN105603569AFast crystallizationHigh strengthSuture equipmentsArtificial filament heat treatmentFiberCarbon nanotube
The invention discloses polyhydroxyalkanoate fibers, a preparing method and application thereof. The polyhydroxyalkanoate fibers contain 0.05-2 wt% of carbon nano tubes, and polyhydroxyalkanoate is copolyester of 3-hydroxybutyric acid and 3-hydroxy caproic acid. The carbon nano tubes are added into polyhydroxyalkanoate PHBHHx, it is accidentally found that the crystallization velocity of polyhydroxyalkanoate is greatly increased by the carbon nano tubes, the crystallization time in the spinning and forming process is shortened, the spinning and forming efficiency is improved, and then production cost is reduced. The fibers are suitable for serving as absorbable suture lines.
Owner:TIANJIN POLYTECHNIC UNIV
Arbekacin synthesis method
InactiveCN104447908AMild removal conditionsEasy to separate and purifySugar derivativesSugar derivatives preparationSynthesis methodsHydrazine compound
The present invention relates to an arbekacin synthesis method. According to the arbekacin synthesis method, di-tert-butyl dicarbonate is adopted as a protection agent, tert-butyloxycarbonyl protection is performed on three amino groups on the sites C3, C2' and C6' of 3',4'-dideoxy-3',4' didehydro-kanamycin B, difference between the remaining free amino groups on the site 1 and the site 3' is adopted to directly and selectively introduce the side chain on the amino group on the site 1, the amino-protected 1-tert-butoxy amide-3-hydroxybutyric acid is directly adopted as an acylation reagent of the amino group on the site 1, and hydrolysis with an acid is adopted to remove the tert-butyloxycarbonyl protection. According to the present invention, the operations of the method are simple, the reaction condition and the protection group removing condition are mild, the separation purification of the product obtained from the reaction is easy compared with the separation purification of the product obtained by adopting other types of the amino acid protection agents, the one-pot reaction is adopted, the concurrent deprotection is adopted, the product yield is high, the production cost is reduced, the industrial production is easily achieved, and hydrazine hydrate and other hazardous compounds are not used so as to provide the advantages of low environment pollution.
Owner:CHANGZHOU FANGYUAN PHARMA
Magnesium borate crystal whisker super induced biomaterial and preparation method thereof
InactiveCN106496782AImprove impact performanceImprove aging resistanceShock resistanceMethyl benzoate
The invention discloses a magnesium borate crystal whisker super induced biomaterial and preparation method thereof, the biomaterial uses polypropylene, poly glycolic acid, and methyl benzoate as the main component, through adding poly-3-hydroxybutyric acid, trihydroxymethyl aminomethane, 2-thiol benzimidazole, di-amino-maleic acid, elhylene diamine tetraacetic acid, dodecyl trimethyl ammonium iodide, epoxidized soybean oil, naphthylacetic acid sodium, magnesium borate whisker, montmorillonite powder, light mass calcium carbonate, dispersant, and stabilizer, supplemented with mixing and stirring, high temperature sintering, crushing, high pressure calcinations, acid soaking, ultrasound dispersing, screw rod extruding, injection treatment and other processes, is prepared fully. The biomaterial super induced with magnesium borate crystal whisker has high low temperature shock resistance ability and high anti-aging property, can satisfy the requirements of each branch, and has relatively good application prospect.
Owner:金福兴
Nitrilase as well as preparation method and application thereof
ActiveCN102031247AImprove stabilityMild reaction conditionsHydrolasesMicroorganism based processesSolubilityAmino acid
The invention discloses a nitrilase as well as a preparation method and application thereof. The nitrilase provided in the invention has the amino acid sequence shown as SEQ ID NO: 20. The nitrilase can be obtained through fermenting Arthrobacternitroguajacolicus bacteria and can be used for hydrolyzing 3-hydroxybutyronitrile to produce 3-hydroxybutyric acid. The reaction has less hydrolysis by-products, mild condition and high space time yield, in addition, the 3-hydroxybutyronitrile has favorable water solubility, and the reaction can be carried out in a pure water phase and facilitate the enzyme stability, therefore, the nitrilase has very high industrialized production potential.
Owner:SHANGHAI PESTICIDE RES INST +1
Recombinant ketoreductase and application thereof to preparation of (R)-3-hydroxybutyric acid and salt thereof
ActiveCN109852593AIncrease vitalitySuitable for industrial productionBacteriaOxidoreductasesHydroxybutyric acidAcetic acid
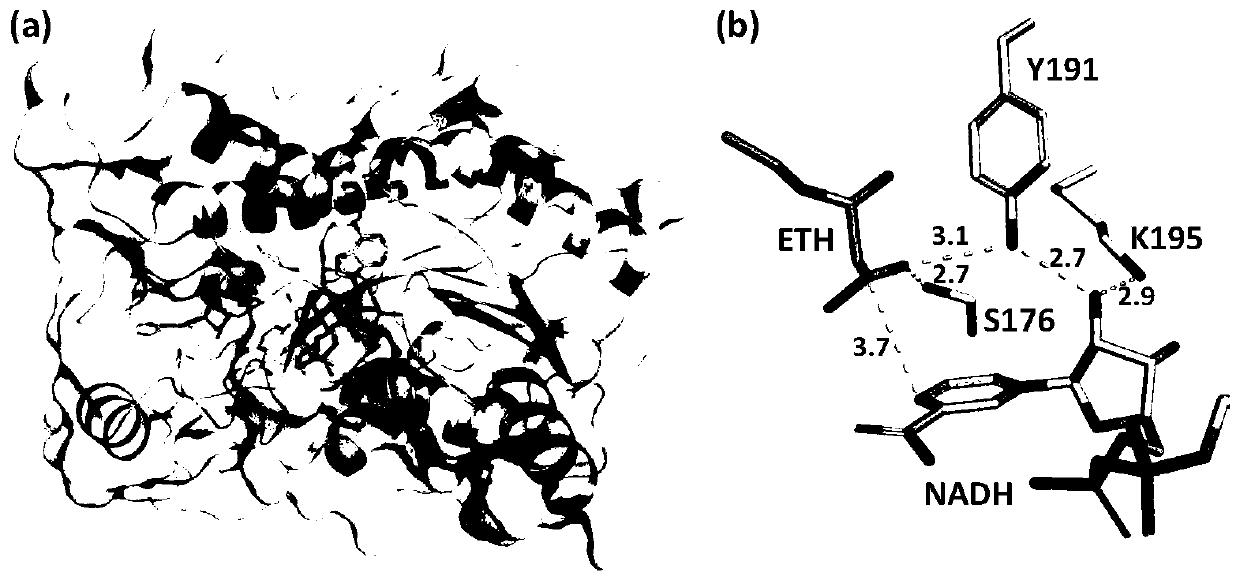
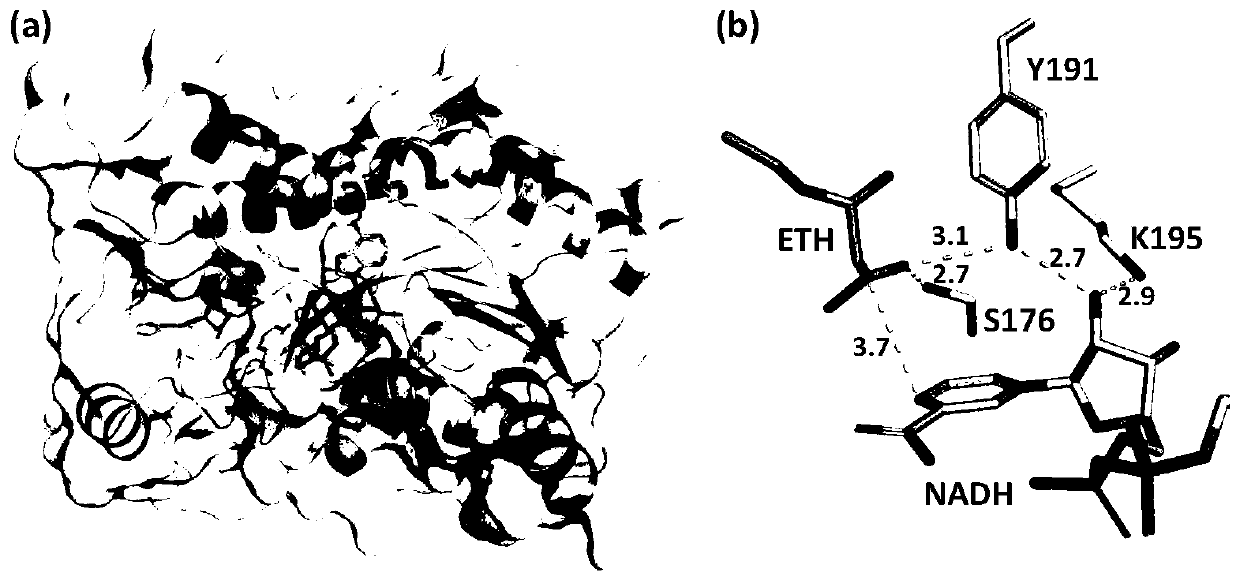
The invention relates to recombinant protein, in particularly to recombinant ketoreductase and an application thereof to preparation of (R)-3-hydroxybutyric acid and salt thereof. The recombinant ketoreductase contains (1) an amino acid sequence as shown in SEQID NO:1, wherein one or more equivalent amino acid residues in the 176th amino acid, the 191st amino acid and the 195th amino acid are substituted; or (2) an amino acid sequence as shown in (1), wherein one or more amino acids except those at the 176th site, the 191st site and the 195th site are substituted, deleted, added and / or inserted. Compared with wild type ketoreductase, the recombinant ketoreductase has the advantage that the enzyme activity is increased. The activity of the recombinant ketoreductase on ethyl acetoacetate substrates is improved by at least 300% than that of the wild type ketoreductase, wherein the activity of a CmCR-186 mutant on the ethyl acetoacetate substrates is improved by 879% than that of the wildtype ketoreductase.
Owner:洛阳华荣生物技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com