Nitrilase as well as preparation method and application thereof
A technology of nitrilase and hydroxybutyronitrile, which is applied in the field of enzymology, can solve the problems of expensive metal catalysts, harsh reaction conditions, and high equipment requirements, and achieve high industrial production potential, mild reaction conditions, and less hydrolysis by-products.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 , Fermentation strain culture and fermentation liquid detection
[0059] 1.1. Fermentation strain culture
[0060] Pick Arthrobacter nitroguajacolicus CGMCC No.2405, inoculate it into the slant medium, and cultivate it at 28°C for 4 days; then, pick the cultured strain from the slant, transfer it to the fermentation medium, and cultivate it at 220rpm and 28°C for 3-4 days , to obtain the fermentation broth.
[0061] 1.2. Stability detection of enzyme solution
[0062] Take 25ml of the fermentation broth obtained in step 1.1, concentrate it 5 times, use 10mM Tris-HCl buffer to suspend the cells, and perform cell disruption. The cell disruption conditions are as follows:
[0063] Ultrasound for 2 seconds, intermittent for 5 seconds, power 700-800W, time 0.5 hours.
[0064] The broken cell solution was centrifuged at 15,000 rpm for 30-40 minutes, and the upper layer of crude enzyme solution was collected.
[0065] After the collected centrifuged supernatant...
Embodiment 2
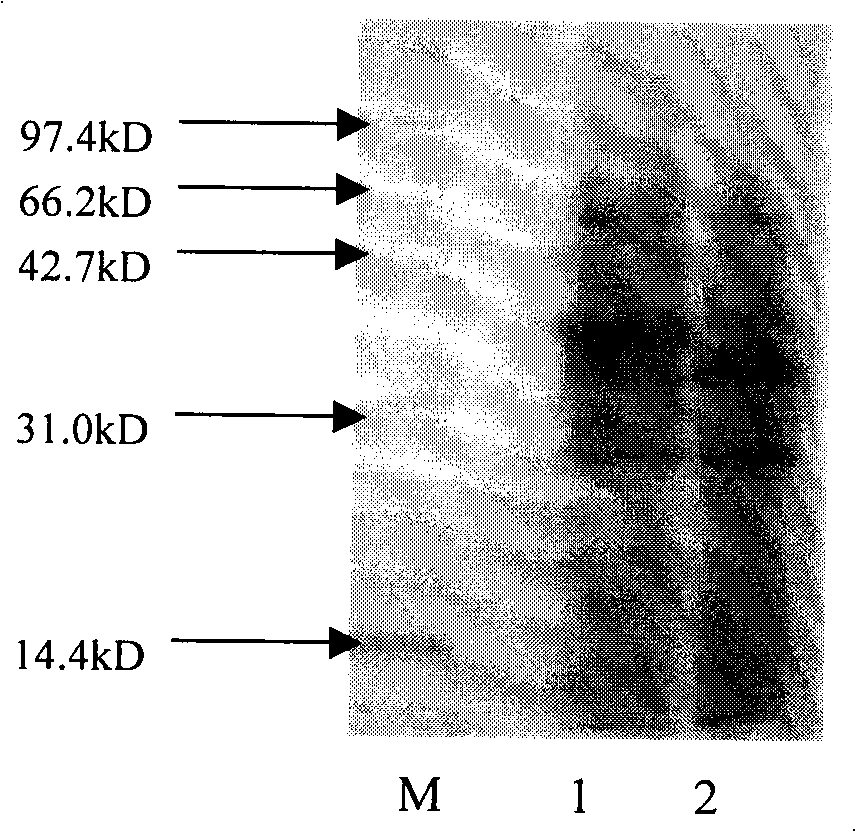
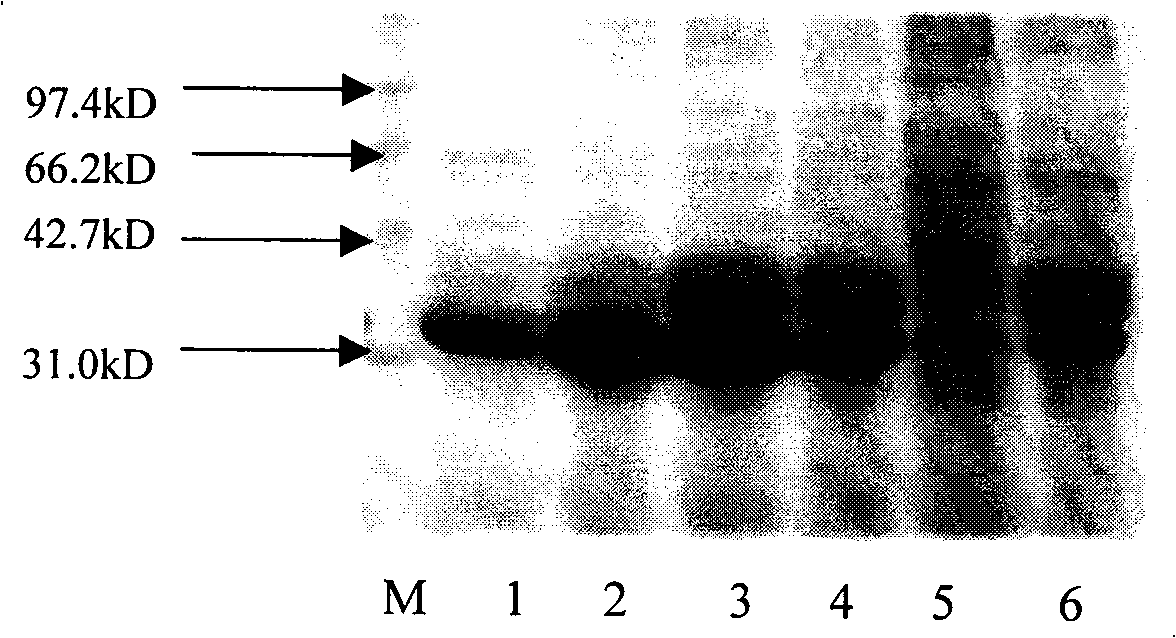
[0073] Example 2 ,protein purification
[0074] The crude enzyme solution in the upper layer obtained in Example 1 was subjected to fractional precipitation with saturated ammonium sulfate, and the precipitate at 0-35% saturation was collected.
[0075] Then, dissolve the crude enzyme precipitate with 0.5M pH7.2 potassium phosphate buffer solution, purify the obtained crude enzyme solution through a phenyl hydrophobic column, collect the eluate, and detect the enzyme activity and protein content of the eluate at the same time, and then The collected fraction with higher specific enzyme activity was purified using a butyl hydrophobic column, and the specific purification conditions were as follows:
[0076] 1. Phenyl hydrophobic column (Phenyl-650C)
[0077] Column bed: height 12cm×diameter 1cm, volume 9.4ml;
[0078] Flow rate: 0.1ml / min adsorption, 1ml / min washing column (0.5M potassium phosphate buffer, pH7.2), 0.1ml / min elution (deionized water);
[0079] Column volume...
Embodiment 3
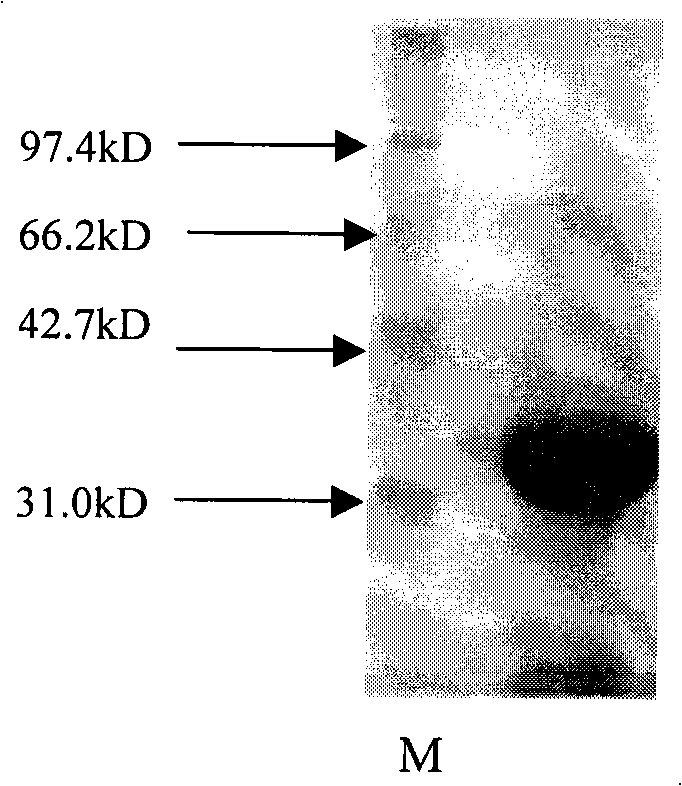
[0097] Example 3 , protein detection
[0098] 3.1. N-terminal amino acid sequence determination
[0099] The N-terminal sequencing of the enzyme obtained by purifying the butyl hydrophobic column in Example 2 showed that the sequence of twenty amino acids at the N-terminal of the enzyme was NH 2 -THPQ FKAAVVQAAPVFLNLD .
[0100] Then, the determined sequence was compared with Blastp, and the result of the comparison showed that the amino acid sequence of the enzyme obtained in Example 2 had a high homology with the nitrilase, therefore, it might be the target protein-nitrilase.
[0101] 3.2. Determination of full-length DNA sequence
[0102] The genome of the strain obtained in Example 1.1 was extracted with reference to the experimental method in "Refined Molecular Biology Experiment Guide (Fourth Edition)" (Science Press).
[0103] The strain genome and vector pUC18 plasmid (purchased from Shanghai Sangong) were completely digested with BamHI, the digested genome and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com