1,3-O-diferuloyl-2-methoxypropanediol as well as preparation method and application thereof
A methanol and dichloromethane technology, applied in the field of 1,3-O-diferulate-2-methoxypropanediol and its preparation and application, can solve the problem of rare non-alkaloid compounds and achieve strong Inhibitory effect, simple preparation method, and wide-ranging sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
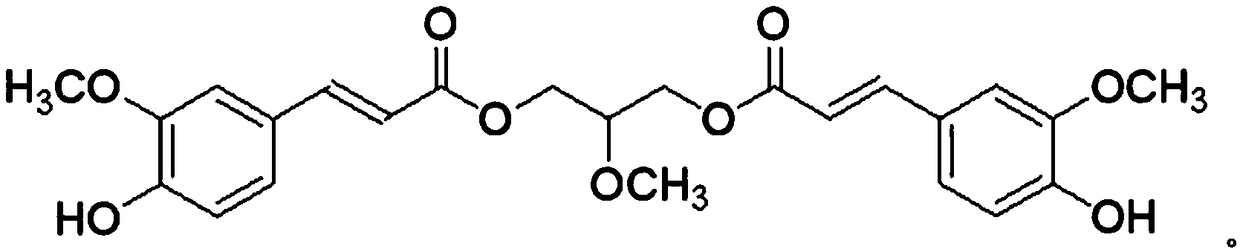
[0021] Example 1: The chemical structural formula of the 1,3-O-diferuloyl-2-methoxypropanediol is
[0022]
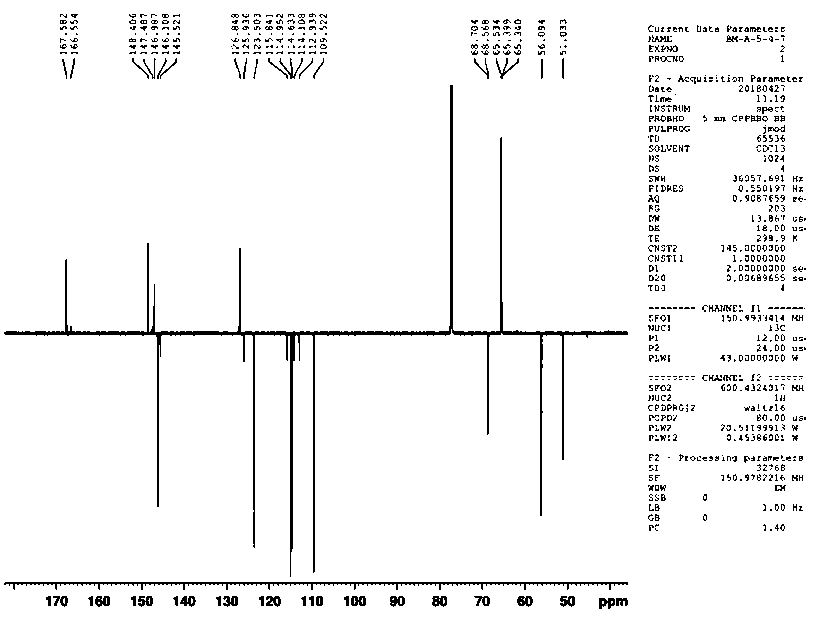
[0023] 1,3-O-diferuloyl-2-methoxypropanediol of the present invention carries out proton nuclear magnetic resonance spectrum ( 1 H-NMR) and carbon nuclear magnetic resonance ( 13 C-APT) analysis, 1 H-NMR spectrum as figure 1 as shown, 13 C-APT spectrum as figure 2 shown, yes figure 1 with figure 2 To analyze the spectrum, the figure 1 with figure 2 The peaks are assigned, figure 1 with figure 2 The peak assignments are shown in Table 1. pass figure 1 , figure 2 From the data in Table 1, it can be seen that the chemical structural formula of 1,3-O-diferuloyl-2-methoxypropanediol of the present invention is shown in the above examples, and it is easily soluble in chloroform and methanol.
Embodiment 2
[0024] Example 2: As an optimization of the above example, it is obtained according to the following method: the first step, after crushing the root of Fritillaria Ili, add methanol, soak it at room temperature for 3 hours to 4 hours, and heat it at 50°C to 60°C Heating and reflux extracting 3 times under the conditions, each time for 1 hour to 3 hours, combining each reflux extract and recovering and concentrating under reduced pressure to obtain the total extract of Fritillaria Ili; the second step is to disperse the total extract of Fritillaria Ili with water After forming a suspension, extract with petroleum ether, chloroform, and ethyl acetate in sequence, and concentrate the extract to obtain the chloroform part extract; the third step, take the chloroform part extract and separate it by gradient elution with silica gel column chromatography to obtain 11 Fraction, wherein, the silica gel column chromatography gradient eluent comprises sherwood oil, methylene dichloride an...
Embodiment 3
[0025] Example 3: As an optimization of the above example, in the first step, add 8ml to 10ml of methanol per 1g of the root of Fritillaria Ili.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com