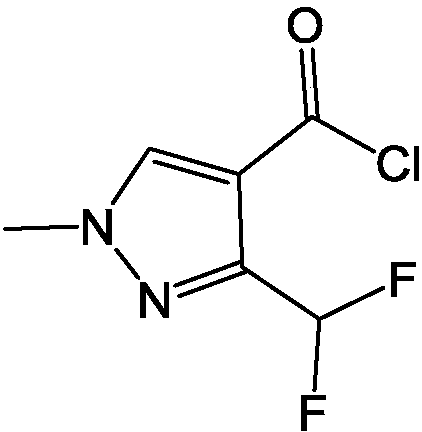
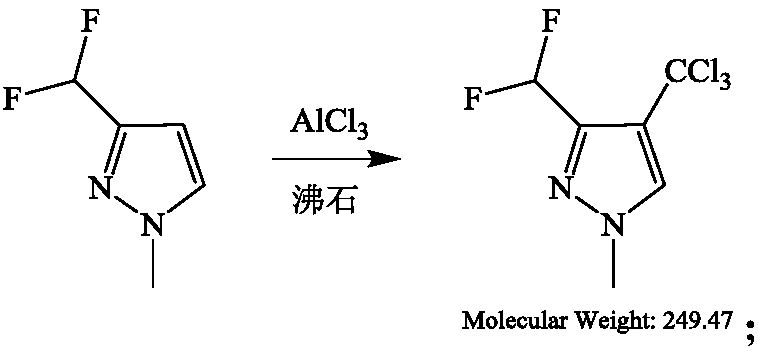
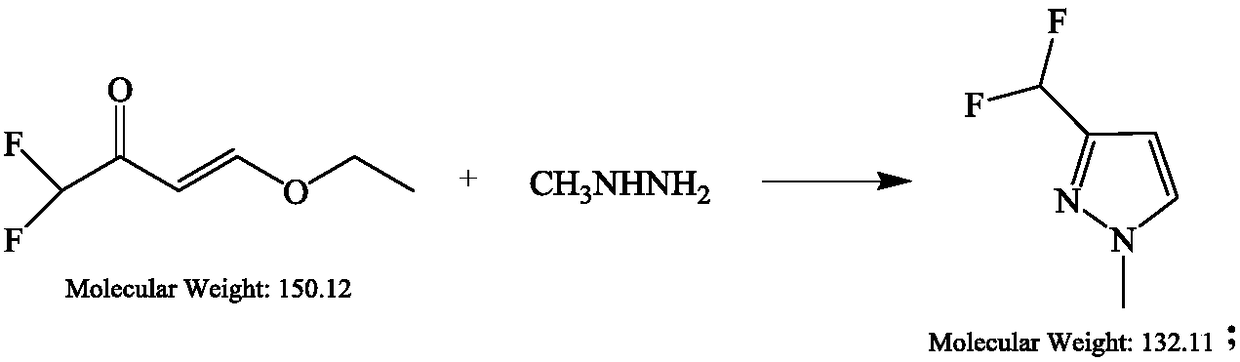Preparation method of 3-difluoromethyl-1-methyl-pyridine-4-carbonyl chloride
A technology of difluoromethyl and carbonyl chloride, which is applied in the field of pharmaceutical chemical synthesis, can solve problems such as large environmental pollution, difficult reaction control, and harsh conditions, and achieve the effects of reducing operational pollution, high synthesis yield, and fewer synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
[0026] At 22°C, 45g of acetic acid solution containing 80% 1,1-difluoro-4-ethoxy-3-buten-2-one (0.24mol) was added dropwise to 20% methylhydrazine aqueous solution , the aqueous solution of methylhydrazine is 99% pure, 11.6g, 0.24mol. The resulting reaction solution was stirred at 22° C. for 19 hours in 560 ml of acetic acid. Acetic acid was then removed under reduced pressure. The oily residue was dissolved in 300 ml methyl tert-butyl ether (MTBE) and washed with 250 ml water. The aqueous phase was extracted once with 150 ml MTBE. The collected organic solutions were dried over sodium sulfate, filtered and the solvent was removed under reduced pressure (40° C. / 400 to 30 mbar). The 23.5 g residue was subjected to fractional distillation to isolate the product. 21.5 g of the main fraction (conversion temperature 52°C, 22 mbar) contained 3-difluoromethyl-1-methyl-pyridine and 5-difluoromethyl-1-methyl-pyridine in a ratio of 3:1. 3-Difluoromethyl-1-pyridine The overall yield...
specific Embodiment 2
[0027] At 22°C, in 45g of acetic acid solution containing 80% of 1,1-difluoro-4-ethoxy-3-buten-2-one (0.24mol), add dropwise to 460ml of methylhydrazineacetic acid solution (99% pure, 12.2 g, 0.26 mol). Under these conditions, the reaction was incubated for 19 hours. The mixture was freed of acetic acid under reduced pressure. The residual oily mixture was dissolved in methyl tert-butyl ether (MTBE, 300ml) and washed with water (250ml). The aqueous phase was extracted once with MTBE (150ml). The collected organic solutions were dried over sodium sulfate, filtered and the solvent was removed under reduced pressure. The residue (23.5 g) was subjected to fractional distillation to isolate the product. The main fraction was distilled under reduced pressure to obtain 21.5 g containing 3-difluoromethyl-1-methyl-pyridine and 5-difluoromethyl-1-methyl-pyridine in a ratio of 4:1 (GC retention time: 5- isomer: 7.6 minutes; 3-isomer: 9.2 minutes). The overall yield of 3-difluoromet...
specific Embodiment 3
[0028] At 22°C, in 45g of acetic acid solution containing 80% of 1,1-difluoro-4-ethoxy-3-buten-2-one (0.24mol), add dropwise to 460ml of methylhydrazineacetic acid solution (99% pure, 14.4 g, 0.31 mol). Under these conditions, the reaction was incubated for 19 hours. The mixture was freed of acetic acid under reduced pressure. The residual oily mixture was dissolved in methyl tert-butyl ether (MTBE, 300ml) and washed with water (250ml). The aqueous phase was extracted once with MTBE (150ml). The collected organic solutions were dried over sodium sulfate, filtered and the solvent was removed under reduced pressure. The residue (23.5 g) was subjected to fractional distillation to isolate the product. The main fraction was distilled under reduced pressure to obtain 21.5 g containing 3-difluoromethyl-1-methyl-pyridine and 5-difluoromethyl-1-methyl-pyridine in a ratio of 4:1 (GC retention time: 5- isomer: 7.6 minutes; 3-isomer: 9.2 minutes). The overall yield of 3-difluoromet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com