A kind of benzothiazole derivative and its preparation method and application
A technology of benzothiazole and derivatives, applied in the field of heterocyclic compounds and fluorescent probes, can solve the problems of rare probes and few reports, achieve good cell membrane penetration, reduce interference, and reduce errors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation of 1,6-(2-(benzothiazol-2-yl)vinyl)naphthalene-2-ol (BTNO):
[0027]
[0028] (1) 6-hydroxy-2-naphthaldehyde (10mmol, 1.7218g), 2-methylbenzothiazole (15mmol, 1.89mL), trimethylchlorosilane (50mmol, 6.40mL), 10mL CH2Cl2 mixed The solution was reacted in a high-pressure reactor at 105°C for 24h. The solvent was removed under reduced pressure, and the resulting solid was dissolved in water and washed with Na 2 CO 3 Adjust the pH of the solution to 6.0 with CH 2 Cl 2 extraction. Remove the solvent to obtain the product crude product;
[0029] (2) the product crude product is separated and purified through a silica gel column, v 乙酸乙酯 :v 正己烷 =1:6 was the eluent, and the product BTNO (1.58 g, 52%) was obtained as a reddish-brown solid. 1 H NMR(400MHz,DMSO)δ10.62(s,1H),10.34(s,1H),10.04(s,1H),9.97(s,1H),9.65(s,1H),8.43(s,1H) ,8.02(d,J=8.7Hz,2H),7.80(dd,J=18.1,8.5Hz,2H),7.21(d,J=8.4Hz,2H),7.02(s,1H). 13 C NMR (101MHz, d 6 -DMSO)δ197.80(s), 197.53(s), 1...
Embodiment 2
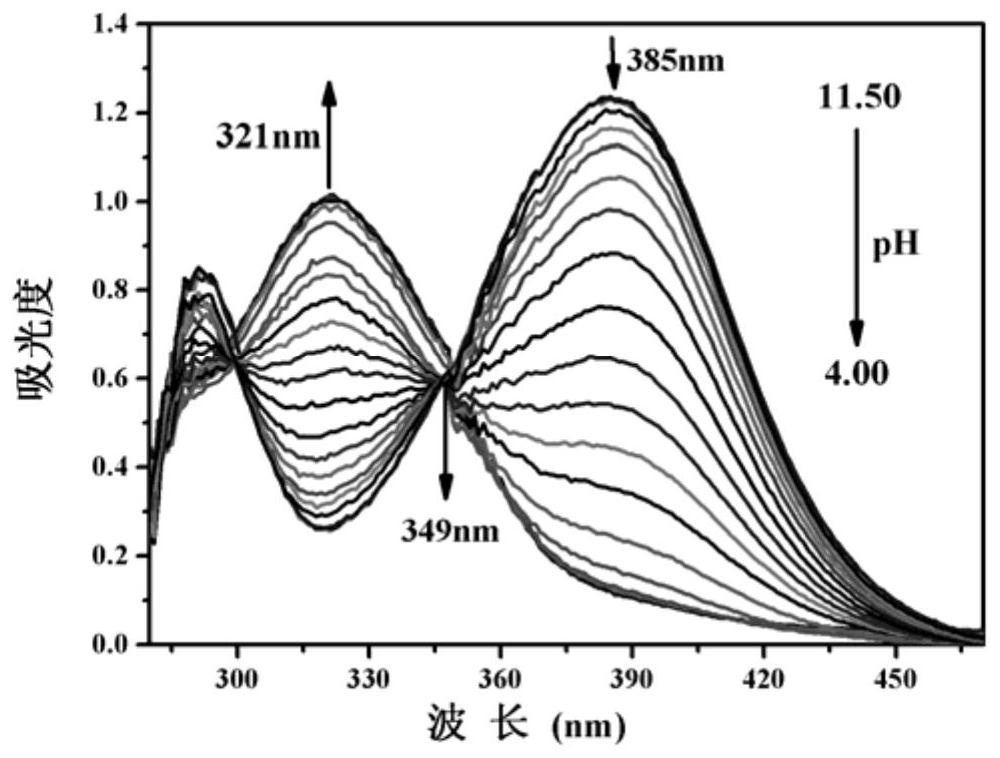
[0031] The BTNO concentration of Example 1 is maintained at 150 μM, and the pH value is adjusted with high-concentration and small-volume HCl and NaOH solutions in the DMSO / water (volume ratio is 2:1) system, and its absorption spectrum is recorded ( figure 1 ). With the decrease of pH value, the absorption peak at 385nm decreases gradually, the absorption peak at 321nm increases correspondingly, and there is an isosbestic point at 349nm. The color of the solution also changed from yellow to light green ( figure 2 ).
Embodiment 3
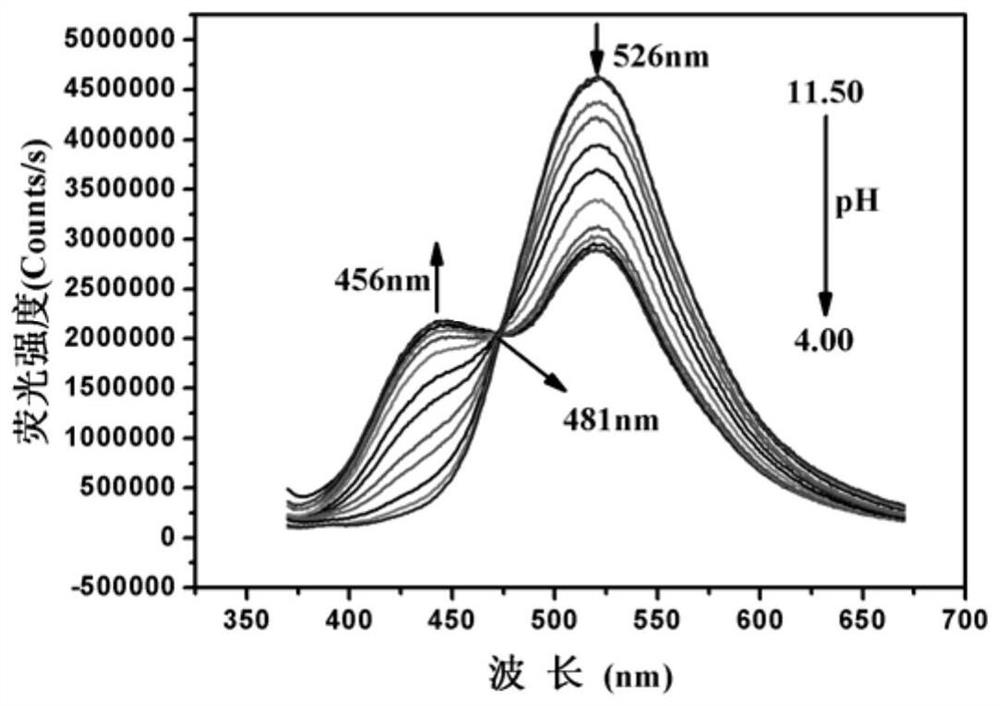
[0033] The BTNO concentration of Example 1 was kept at 10 μM, and the pH value was adjusted with high-concentration and small-volume HCl and NaOH solutions in the DMSO / water (volume ratio: 2:1) system, and the fluorescence emission was recorded at 349 nm as the excitation wavelength. spectrum( image 3 ). With the decrease of pH value, the fluorescence peak at 526nm weakens gradually, while the fluorescence peak at 456nm increases gradually. The color of the solution changed from green to blue ( Figure 4 ). Fitting F via the Boltzmann function 456nm / F 526nmValue vs. pH change curve, calculate pK a The value is 7.91±0.034 ( Figure 5 ), the pH response linear range is 7.00-9.50. The linear regression equation is F 456nm / F 526nm =2.13641-0.21119×pH, correlation coefficient R 2 =0.9995( Image 6 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com