Synthesis method of 4-chlorothiophene-2-carbonyl derivative
A synthesis method, the technology of chlorothiophene, applied in the direction of organic chemistry, etc., can solve the problems of high post-processing cost, low yield, strong combustion support, etc., and achieve the effects of easy post-processing and purification, reduced production procedures, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1: Synthesis of 2-acetyl-4-chlorothiophene
[0036]
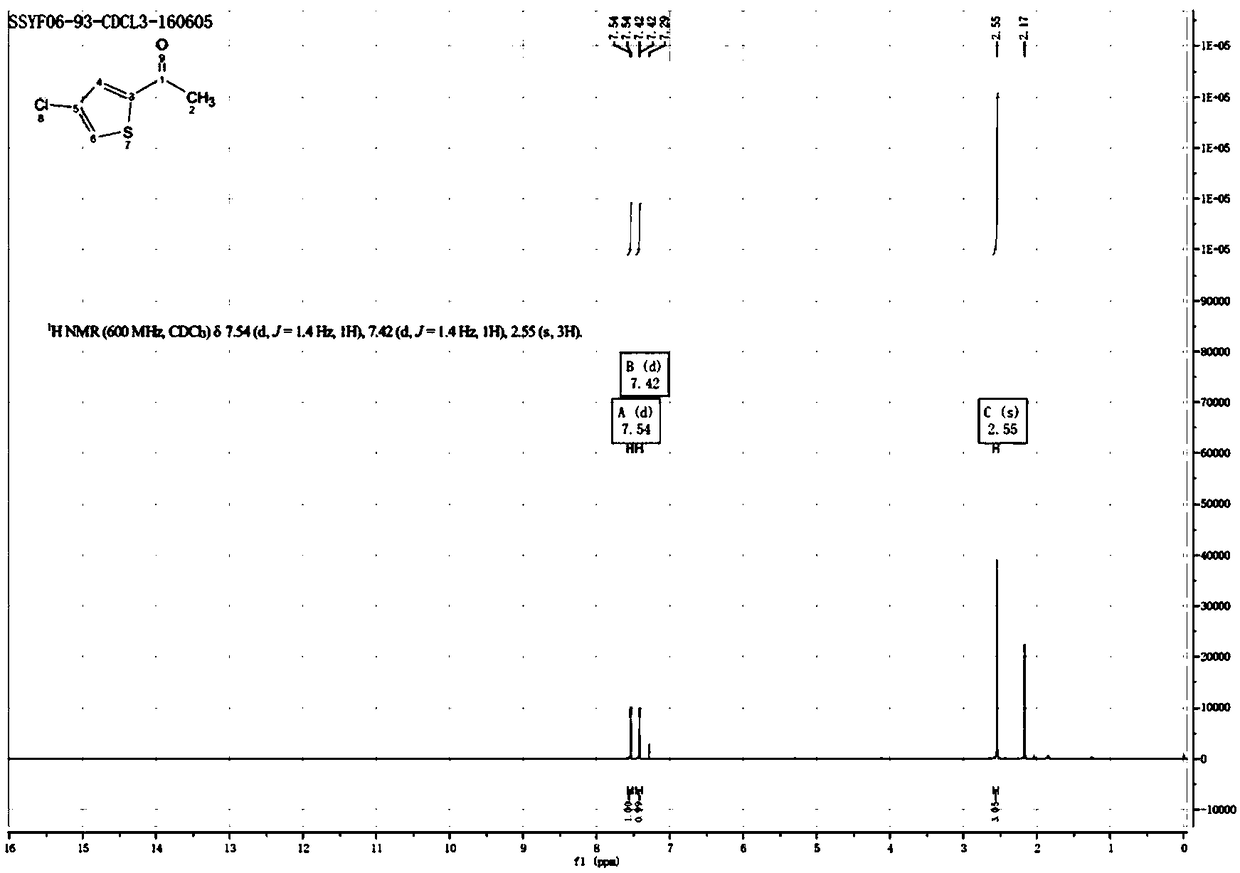
[0037] The reaction formula is shown in formula (II). Dissolve 200g of 2-acetylthiophene Sm1 (1eq) in 2L of dichloromethane, add 147.3g of trichloroisocyanuric acid Sm2 (0.4eq), and slowly divide at 10-15°C. 1060 g of aluminum trichloride (5 eq) was added in batches and the addition was completed within 2 hours. Then react at 10-15°C for 2 hours, TLC detects that the reaction is complete, pour the reaction solution into 2L of ice-cold 1M hydrochloric acid solution, extract with dichloromethane, dry the organic phase with sodium sulfate, concentrate and distill to obtain 210g of 2-acetyl-4- Chlorothiophene, the yield is 82.48%, and the proton nuclear magnetic spectrum is as figure 1 shown.
Embodiment 2
[0038] Embodiment 2: the synthesis of 2-formyl-4-chlorothiophene
[0039]
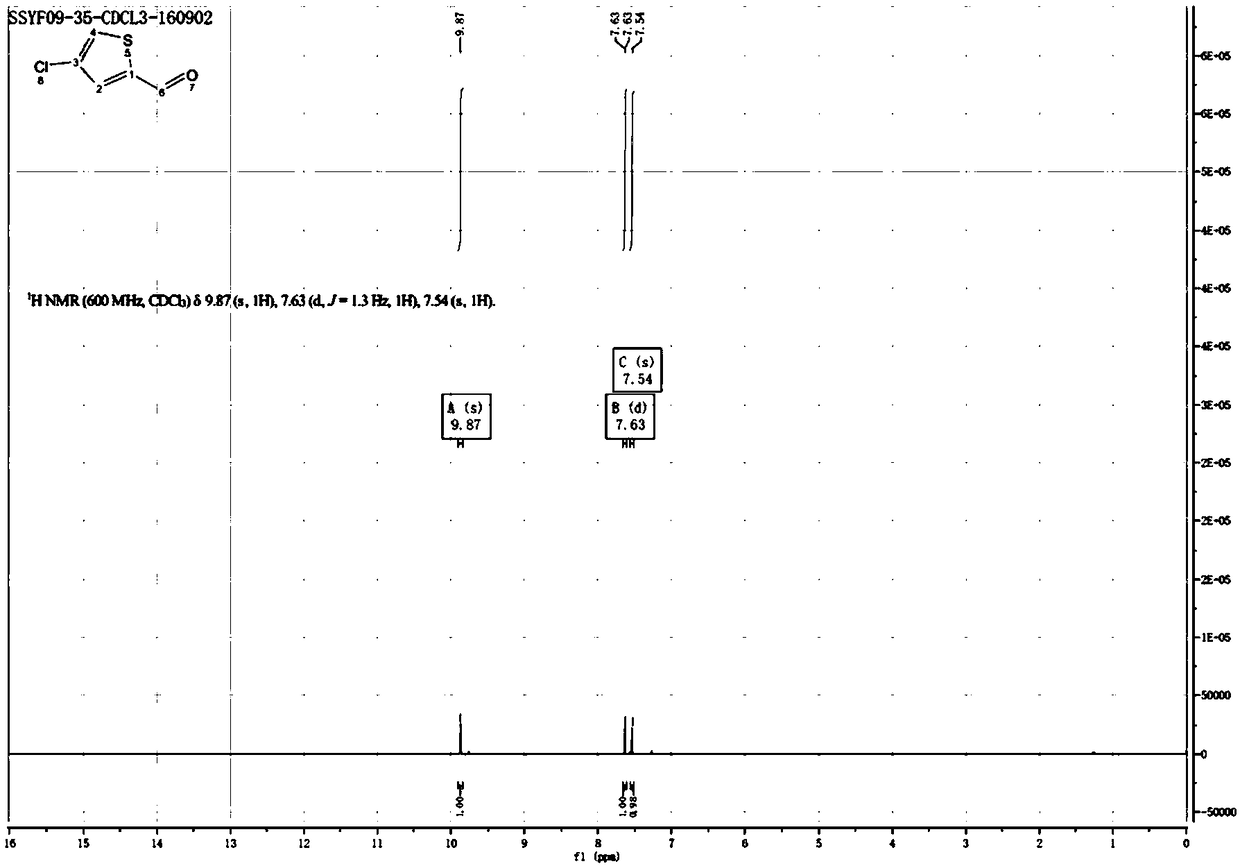
[0040] The reaction formula is shown in formula (III). Dissolve 50g of 2-formylthiophene Sm1 (1eq) in 500ml of dichloromethane, add 41.5g of trichloroisocyanuric acid Sm2 (0.4eq), and slowly 297 g of aluminum trichloride (5 eq) were added in portions over 1 hour. Then react at 10-15°C for 2 hours, TLC detects that the reaction is complete, pour the reaction solution into 500ml of ice-cold 1M hydrochloric acid solution, extract with dichloromethane, dry the organic phase with sodium sulfate, concentrate and distill to obtain 53g of 2-formyl-4- Chlorothiophene, the yield is 81.09%, and the proton nuclear magnetic spectrum is as figure 2 shown.
Embodiment 3
[0041] Embodiment 3: the synthesis of 4-chlorothiophene-2-carboxylic acid
[0042]
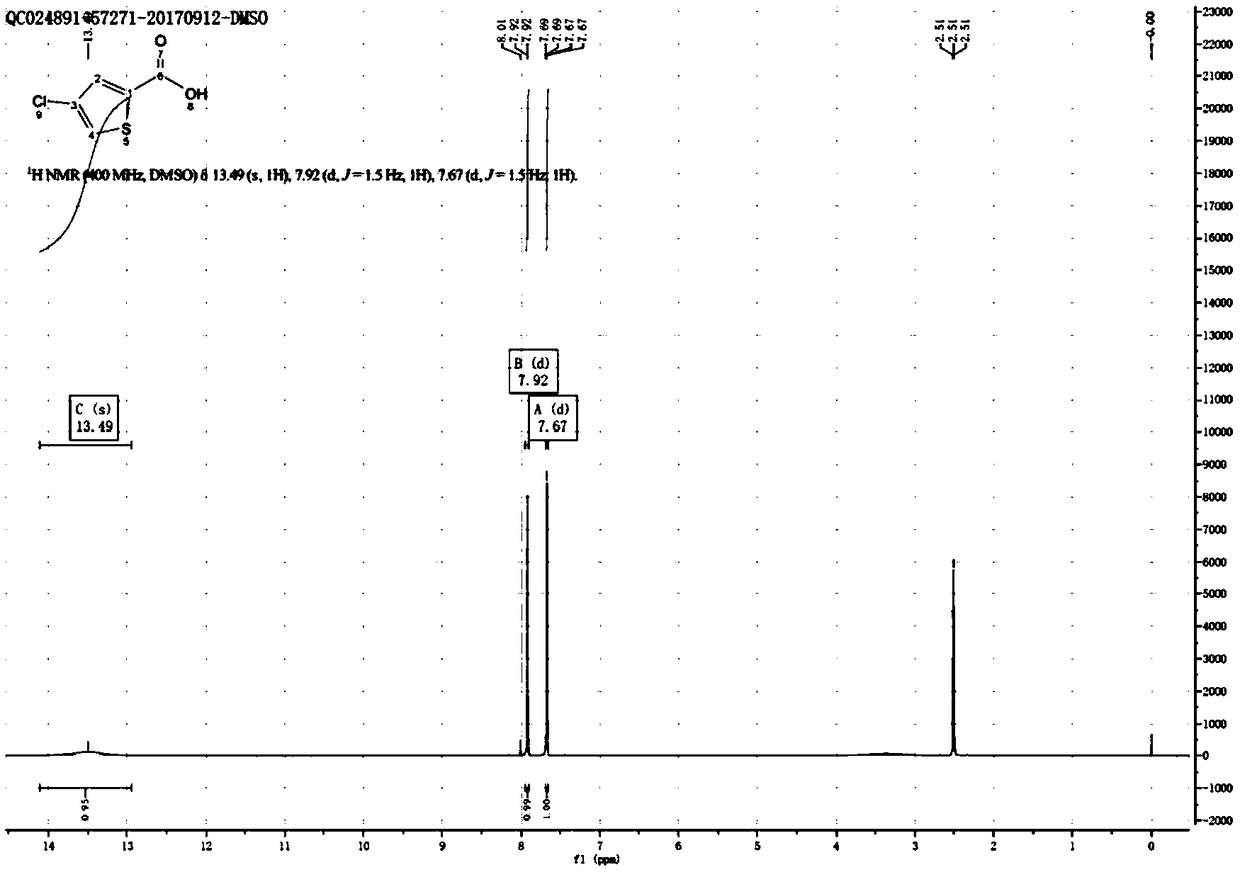
[0043] The reaction formula is shown in formula (IV). Dissolve 20g of thiophene-2-carboxylic acid Sm1 (1eq) in 200ml of dichloromethane, add 14.5g of trichloroisocyanuric acid Sm2 (0.4eq), and slowly Add 104.1g of aluminum trichloride (5eq) in batches and complete the addition within 30min. Then react at 10-15°C for 2 hours, TLC detects that the reaction is complete, wash the reaction solution into 200ml of ice-cold 1M hydrochloric acid solution, extract with dichloromethane, dry the organic phase with sodium sulfate, concentrate and beat with PE to obtain 20.4g of 4-chlorothiophene-2 -Formic acid, the yield is 80.4%, and the proton nuclear magnetic spectrum is as image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com