Semeglutide synthesis method
A technology of semaglutide and peptide resin, applied in the field of synthesizing semaglutide, can solve the problems of affecting downstream purification, waste of raw materials, difficult coupling, etc., so as to improve the purity and yield, reduce the generation of impurities, and reduce the synthesis of semaglutide. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
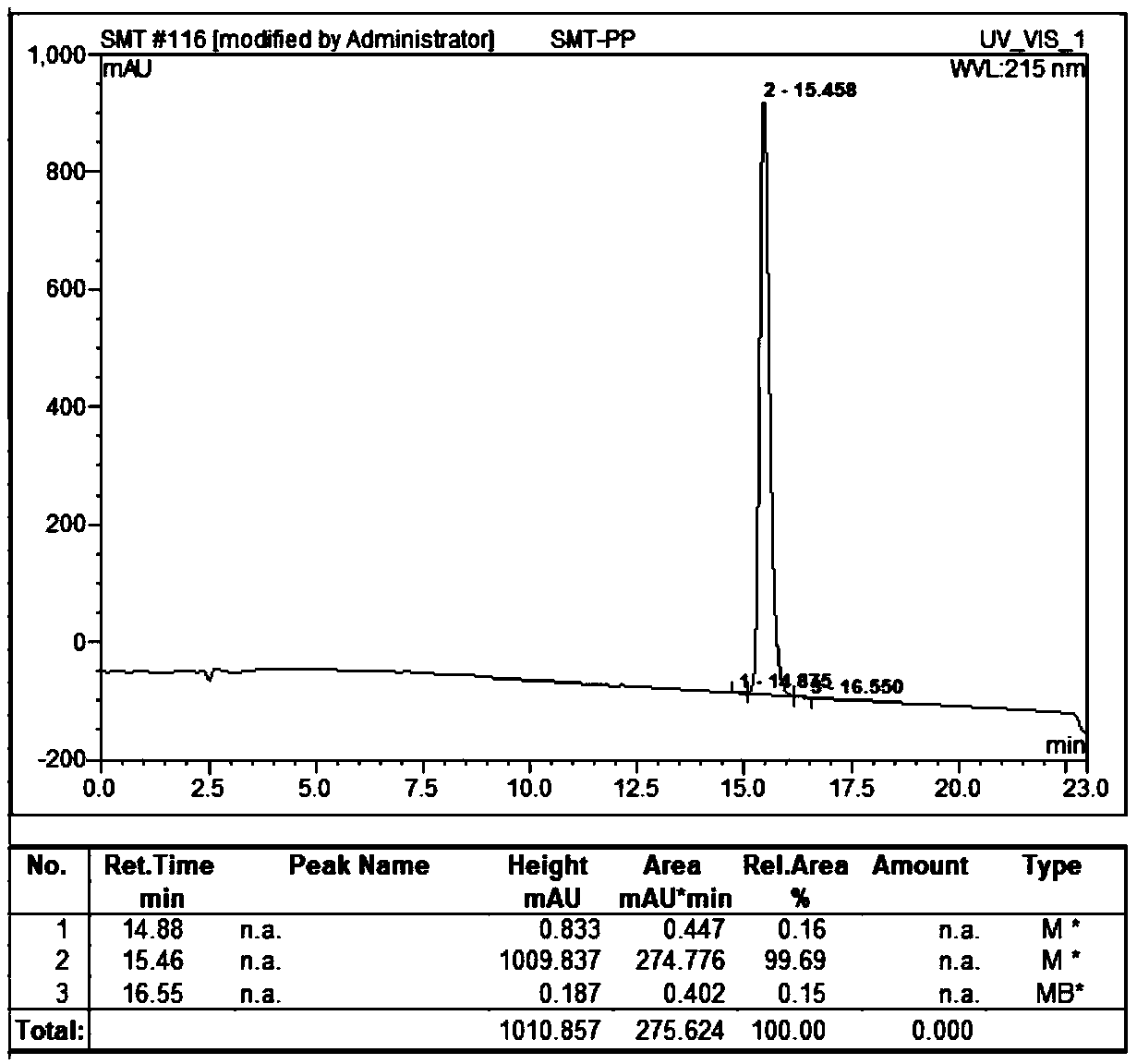
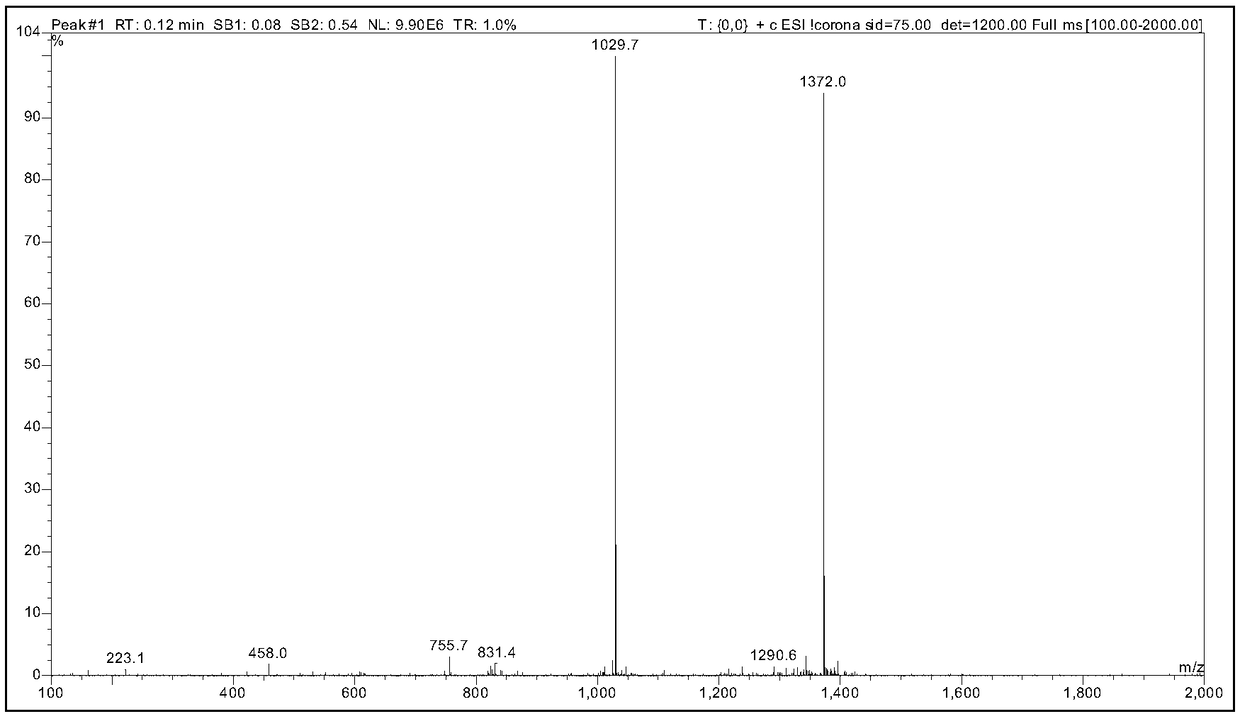
Examples
Embodiment 1
[0050] Embodiment 1: the synthesis of Fmoc-AEEA-AEEA dipeptide fragment
[0051] A. Add 180g of 2-Cl CTC Resin resin with a degree of substitution of 1.0mmol / g into the reactor, add 600ml of DCM to swell for 30min, and drain it. Weigh 69.37g of Fmoc-AEEA into a 1L beaker, add 600ml of DMF solution, and stir with a magnetic stirrer in an ice-water bath. After the amino acid is completely dissolved, add 31.43ml of DIEA, and continue stirring for 5 minutes to activate. Slowly add the above activation solution into the reaction kettle, add 62.87ml after 15min, react at room temperature for 2h, drain the reaction solution, add DMF 600ml to wash once, repeat DMF wash twice, add blocking solution 600ml (volume ratio DCM: MeOH:DIEA=17:2:1) After sealing for 10 min, drain and repeat the sealing once. Add 600ml of DMF to wash for 1 min, then drain and repeat DMF washing twice to obtain Fmoc-AEEA-CTC Resin.
[0052] B. Add 300 ml of 20% piperidine / DMF solution, mix for 15 minutes, and ...
Embodiment 2
[0055] Example 2: Synthesis of fully protected peptides for the main chain of semaglutide
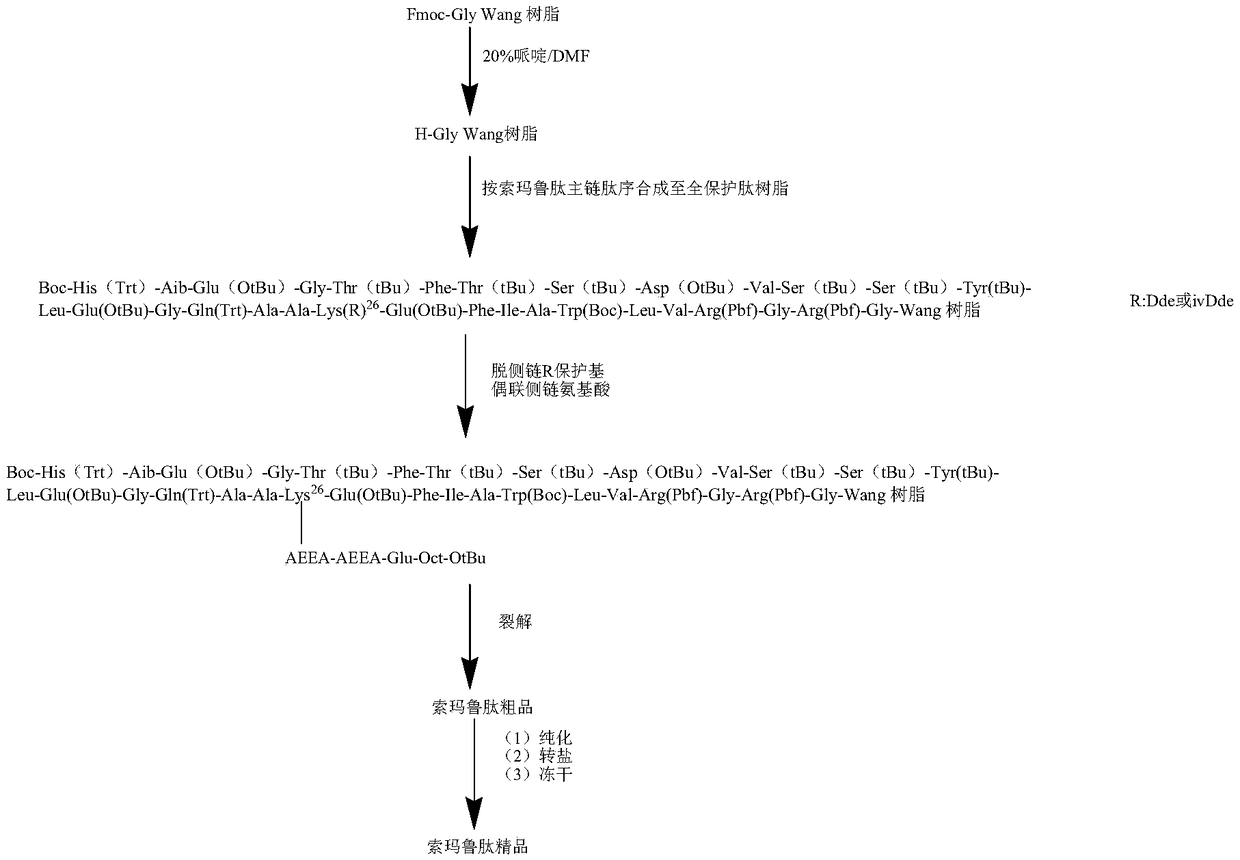
[0056] In this embodiment, the main chain full protection peptide of semaglutide refers to: Boc-His (Trt) 7 -Aib 8 -Glu(OtBu) 9 -Gly 10 -Thr(tBu) 11 -Phe 12 -Thr(tBu) 13 -Ser(tBu) 14 -Asp(OtBu) 15 -Val 16 -Ser(tBu) 17 -Ser(tBu) 18 -Tyr(tBu) 19 -Leu 20 -Glu(OtBu) 21 -Gly 22 -Gln(Trt) 23 -Ala 24 -Ala 25 -Lys(R) 26 -Glu(OtBu) 27 -Phe 28 -Ile 29 -Ala 30 -Trp(Boc) 31 -Leu 32 -Val 33 -Arg(Pbf) 34 -Gly 35 -Arg(Pbf) 36 -Gly 37 -Wang Resin, R is Dde or ivDde.
[0057] A. Add 100g (30mmol) of Fmoc-Gly-Wang Resin with a substitution degree of 0.3mmol / g into the reaction column, add 400mml of dichloromethane to swell for 30min, and drain it. Add 300ml of 20% piperidine / DMF solution, mix for 5min, then drain, add DMF 300ml, wash for 1min, then drain. Add 300 ml of 20% piperidine / DMF solution, mix for 15 minutes, and drain. Add 300ml of DMF to wash for 1min, then drain...
Embodiment 3
[0060] Embodiment 3: Synthesis of fully protected peptide of semaglutide
[0061] In this embodiment, the semaglutide linear chain full protection peptide refers to: Boc-His (Trt) 7 -Aib 8 -Glu(OtBu) 9 -Gly 10 -Thr(tBu) 11 -Phe 12 -Thr(tBu) 13 -Ser(tBu) 14 -Asp(OtBu) 15 -Val 16 -Ser(tBu) 17 -Ser(tBu) 18 -Tyr(tBu) 19 -Leu 20 -Glu(OtBu) 21 -Gly 22 -Gln(Trt) 23 -Ala 24 -Ala 25 -Lys[AEEA-AEEA-Glu(Oct-OtBu)-OtBu] 26 -Glu(OtBu) 27 -Phe 28 -Ile 29 -Ala 30 -Trp(Boc) 31 -Leu 32 -Val 33 -Arg(Pbf) 34 -Gly 35 -Arg(Pbf) 36 -Gly 37 -Wang Resin.
[0062] A. Add 600ml of hydrazine hydrate / DMF or hydrazine hydrate / DCM solution with a volume concentration of 1 to 5% to the fully protected main chain peptide resin obtained in Example 2, remove the protective group for 15 to 30 minutes, drain and repeat the hydrazine hydrate / DMF or hydrazine hydrate / DCM solution removed once. Add 600ml DMF to wash 1 time, repeat DMF wash 4 times to obtain
[0063] Boc-His(Trt) 7 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com