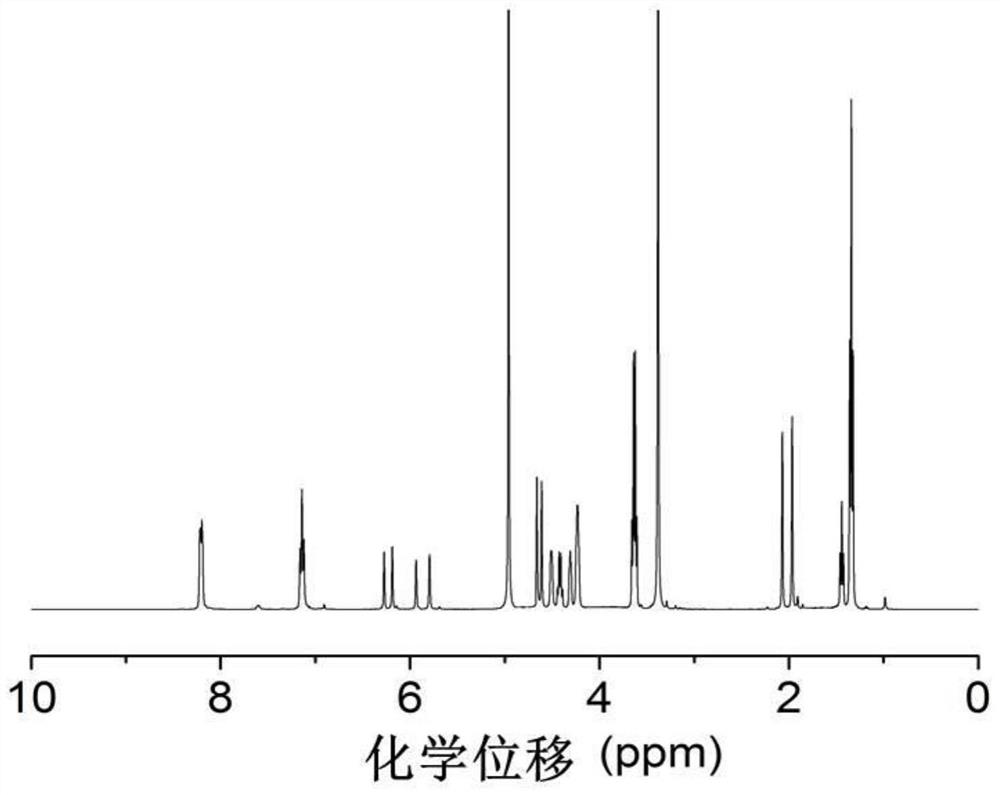
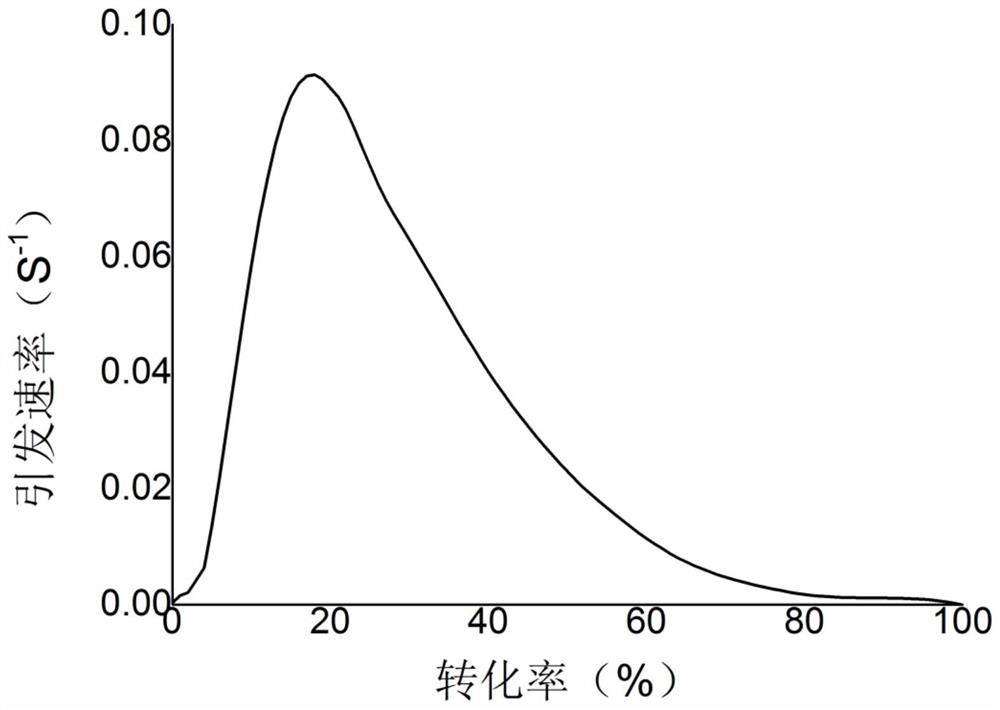
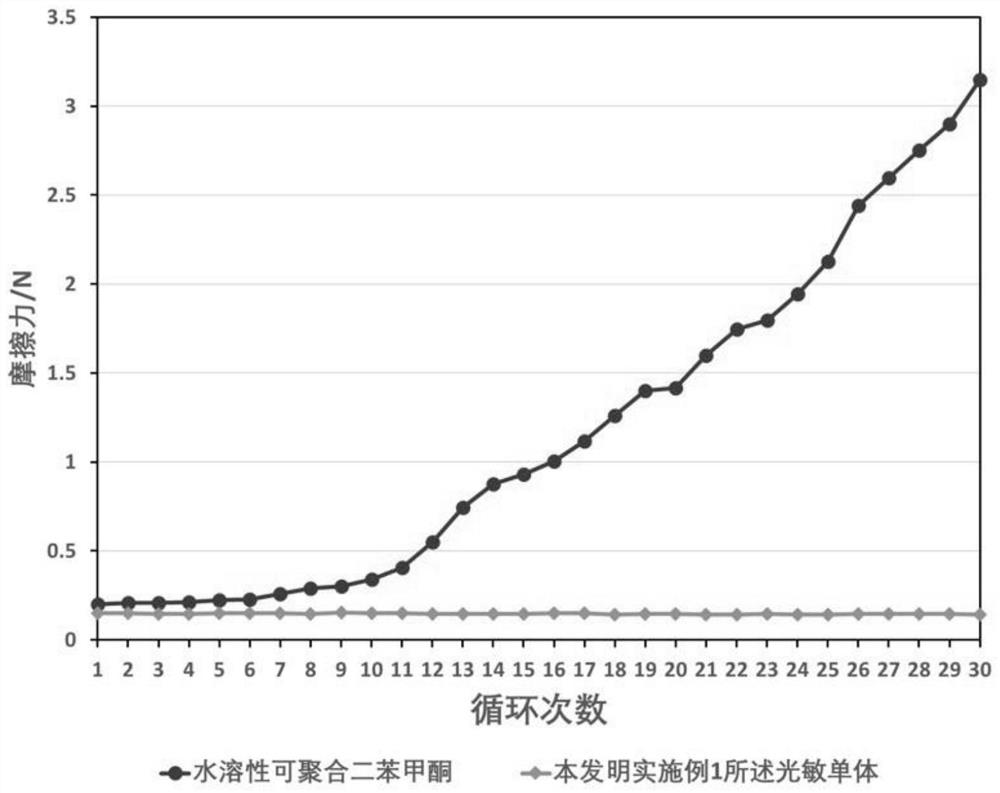
Water-soluble polymerizable photosensitive monomer and its preparation method and application
A water-soluble, photosensitive technology, applied in the field of photocuring, can solve the problems of large molecular weight of photoinitiator, easy migration to the product surface, and high viscosity of prepolymer, so as to reduce migration and volatilization, improve photocuring efficiency and lubricity no drop effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
[0048] The first embodiment of the present invention provides a water-soluble polymerizable photosensitive monomer.
[0049] The photosensitive monomer contains: 1) a unit containing a photosensitive structure; 2) a unit containing a quaternary ammonium salt structure; 3) a unit containing an unsaturated bond structure;
[0050] The unit containing the photosensitive structure is at least connected to the unit containing the quaternary ammonium salt structure through -C(=O)-, and the unit containing the unsaturated bond structure is at least connected to the unit containing the quaternary ammonium salt structure Units containing photosensitive structures are connected.
[0051] Preferably, the unit containing the photosensitive structure is directly connected to the unit containing the quaternary ammonium salt structure through -C(=O)-, and the unit containing the unsaturated bond structure is connected to the unit containing the quaternary ammonium salt structure through the ...
no. 2 approach
[0072] The second embodiment of the present invention provides a method for preparing a water-soluble polymerizable photosensitive monomer.
[0073] The water-soluble polymerizable photosensitive monomer of the present invention is obtained by carrying out acid halide treatment on the molecular end of the compound containing the photosensitive structure, and then reacting with (meth)acrylic esters containing tertiary amino groups.
[0074] Specifically, the following preparation process can be adopted:
[0075] 1) Dissolving the compound containing the photosensitive structure and the acid-binding agent in the organic solvent 1, slowly adding the halogenated alkyl acid halide solution dropwise therein, reacting in an ice bath for a period of time, then rising to room temperature to continue the reaction, and performing post-treatment after the reaction to obtain the compound containing Intermediates of photosensitive structures and halogens;
[0076]2) Dissolve the intermedia...
no. 3 approach
[0096] In the third embodiment of the present invention, the application of the water-soluble polymerizable photosensitive monomer of the present invention in the preparation of a hydrophilic lubricating coating is provided. Hydrophilic coatings of medical devices are generally prepared from photocurable components. The water-soluble polymerizable photosensitive monomer of the present invention can directly be used as a photoinitiator to promote the curing of the coating. Preferably, the water-soluble polymerizable Photosensitive monomers are used to copolymerize with other hydrophilic monomers to obtain photocurable polymers, and then the polymers are used in the preparation of hydrophilic coatings, so that the coating process does not require the addition of small molecule light Initiator, to obtain a hydrophilic coating with excellent lubricating properties and no odor. After repeated friction for 30 times in a simulated human tissue environment, the coating does not fall of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com