Eucheuma blood fat reducing tetrapeptide and application thereof
A technology of Eucheuma and blood lipid lowering, applied in the direction of application, tetrapeptide components, peptides, etc., to achieve good lipid-lowering activity, good blood-lipid-lowering activity, and the effect of reducing fat accumulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
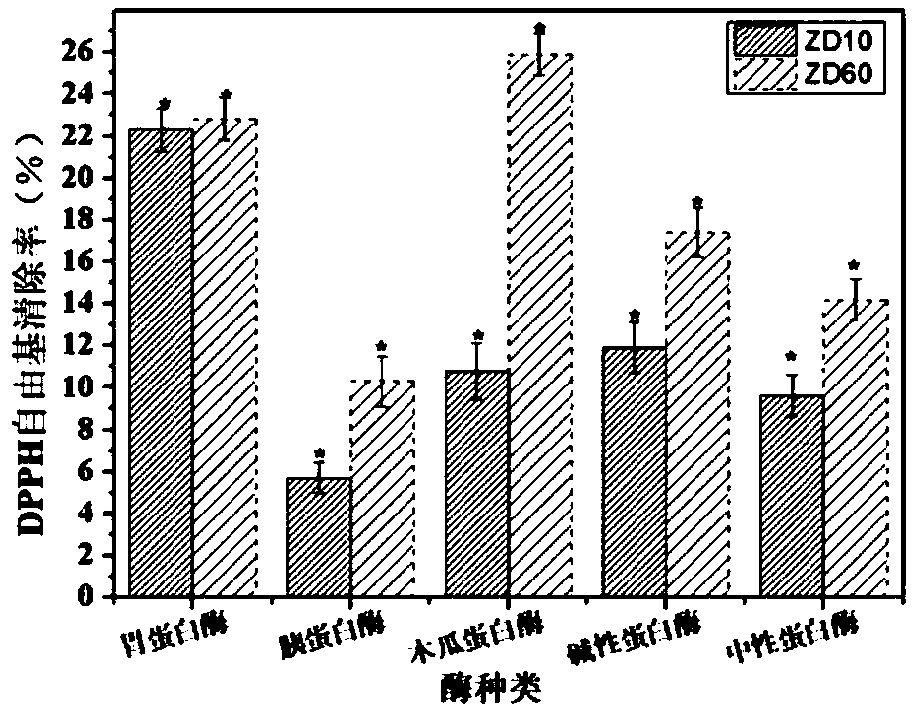
[0031] A blood lipid-lowering tetrapeptide of Eucheuma, the amino acid sequence of which is Phe-Tyr-Lys-Ala (FYKA), the molecular weight determined by ESI-MS is 527.50Da, and has good lipid-lowering activity. The Eucheuma hypolipidemic oligopeptide Phe-Tyr-Lys-Ala (FYKA) Eucheuma tetrapeptide Phe-Tyr-Lys-Ala (FYKA) can significantly reduce the amount of fat accumulation in liver cells induced by oleic acid, significantly reduce the The levels of triglyceride (TG) and total cholesterol (TC) indicated that Phe-Tyr-Lys-Ala (FYKA) had better lipid-lowering activity. Therefore, Phe-Tyr-Lys-Ala (FYKA) has good blood lipid-lowering activity and can be used in the development of blood-lipid-lowering related medicines, health care products, and special medical foods.
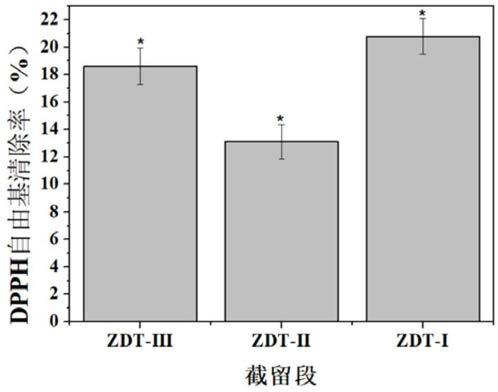
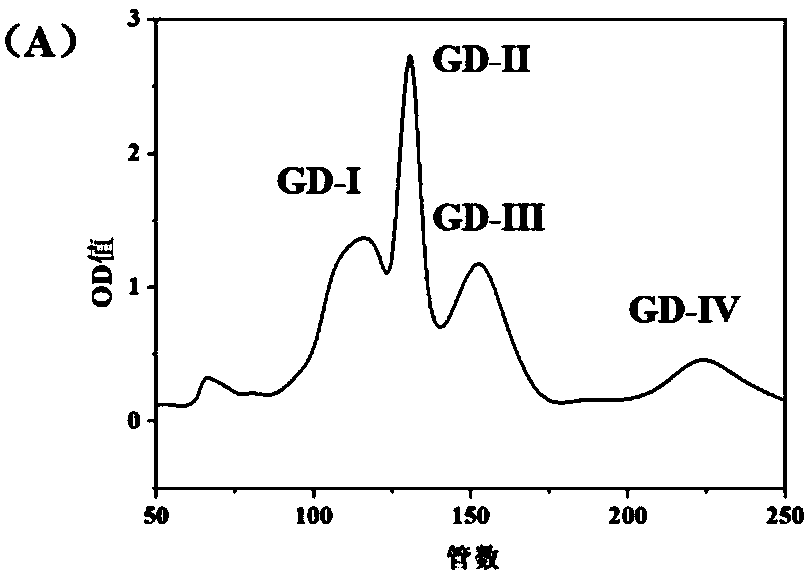
[0032] A preparation method of Eucheuma hypolipidemic tetrapeptide, comprising the preparation of Eucheuma algae protein, the enzymatic hydrolysis of Eucheuma algae protein, and the preparation of Eucheuma anti-oxidation...
Embodiment 2
[0044] A blood lipid-lowering tetrapeptide of Eucheuma, the amino acid sequence of which is Phe-Tyr-Lys-Ala (FYKA), the molecular weight determined by ESI-MS is 527.50Da, and has good lipid-lowering activity.
[0045] A preparation method of Eucheuma hypolipidemic tetrapeptide, comprising the preparation of Eucheuma algae protein, the enzymatic hydrolysis of Eucheuma algae protein, and the preparation of Eucheuma anti-oxidation oligopeptide, wherein the extraction solvent of the Eucheuma algae protein preparation step has a concentration of 0.1% (W / V) CaCl 2 solution, CaCl 2 The solution contains CaCl 2 2.5% ethacridine lactate and 15.0% potassium nitrate by weight.
[0046] The specific steps of the preparation method of the above-mentioned Eucheuma hypolipidemic tetrapeptide are:
[0047] 1) Preparation of Eucheuma algae protein: Take Eucheuma powder, add extraction solvent according to the ratio of material to liquid 1g:100mL and soak for 8h, then use ultrasonic frequenc...
Embodiment 3
[0054] A blood lipid-lowering tetrapeptide of Eucheuma, the amino acid sequence of which is Phe-Tyr-Lys-Ala (FYKA), the molecular weight determined by ESI-MS is 527.50Da, and has good lipid-lowering activity.
[0055]A preparation method of Eucheuma hypolipidemic tetrapeptide, comprising the preparation of Eucheuma algae protein, the enzymatic hydrolysis of Eucheuma algae protein, and the preparation of Eucheuma anti-oxidation oligopeptide, wherein the extraction solvent of the Eucheuma algae protein preparation step has a concentration of 0.5% (W / V) CaCl 2 solution, CaCl 2 The solution contains CaCl 2 3.0% ethacridine lactate and 16.0% potassium nitrate by weight.
[0056] The specific steps of the preparation method of the above-mentioned Eucheuma hypolipidemic tetrapeptide are:
[0057] 1) Preparation of Eucheuma algae protein: Take Eucheuma powder, add extraction solvent according to the ratio of material to liquid 1g:100mL and soak for 12 hours, then use ultrasonic fre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com