Recombinant fusion protein in antibody targeting and application thereof in epigenetics
A fusion protein and protein technology, applied in the biological field, can solve the problems of destruction, false negative, high cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 115
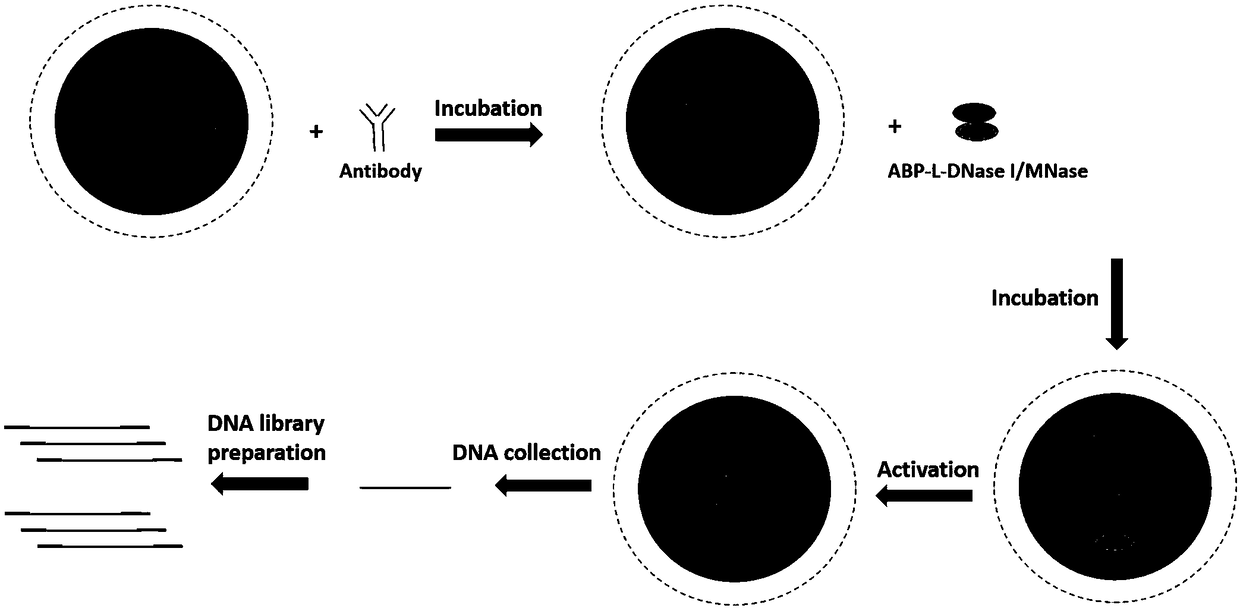
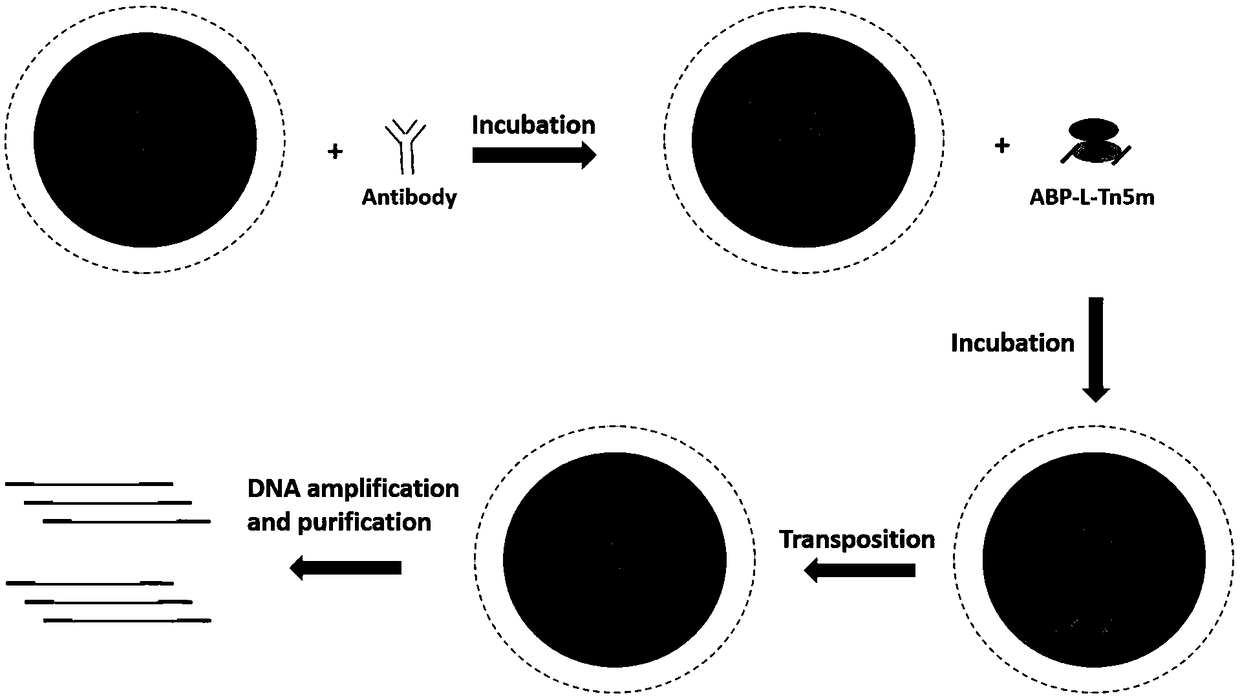
[0076] Example 1 Cloning construction of 15 different ABP-L-DFEs and construction of two DFE-L-ABPs
[0077] In this example, 17 ABP-L-DFE and DFE-L-ABP with different structures were prepared, named ABP-L-DFE-1, ABP-L-DFE-2, ABP-L-DFE-3, ABP respectively -L-DFE-4, ABP-L-DFE-5, ABP-L-DFE-6, ABP-L-DFE-7, ABP-L-DFE-8, ABP-L-DFE-9, ABP-L -DFE-10, ABP-L-DFE-11, ABP-L-DFE-12, ABP-L-DFE-13, ABP-L-DFE-14, ABP-L-DFE-15, DFE-L-ABP -1 and DFE-L-ABP-2.
[0078] 1. All the gene sequences were artificially synthesized, and the gene fragments were respectively cloned into the pET28a vector using a homologous recombination kit (ClonExpress II One StepCloning Kit, Vazyme, C112) to construct a recombinant expression vector.
[0079] (1) A fusion protein called ABP-L-DFE-1, its N-terminal domain is a part of streptococcal G protein (Protein G), and the C-terminal domain is DNAse I, and the two domains are passed through a short Rigid Linker Peptide Linker 1 Ligation.
[0080] The coding nuc...
Embodiment 2
[0201] Example 2 Verification of Antibody Binding Activity of Modified Protein
[0202] Apply the ELISA (enzyme-linked immunosorbent assay) principle to detect the antibody (Ig) binding activity of the fusion protein, dilute the fusion enzyme to be tested to 1-10 μg / mL with coating buffer (50mM carbonate buffer, pH 9.6), The molar concentrations of different fusion enzymes are the same, mix well, add to the microtiter plate, 100 μL / well, each fusion protein has three replicate wells, the negative control does not contain the fusion protein, and incubate overnight at 2-8°C; wash with washing solution (1 *PBST) and washed 3 times; add blocking solution (1*PBS, 5% BSA), 200μL / well, and incubate at 37°C for 2-3h; dilute the enzyme-labeled antibody (1*PBST, 5%BSA) Goat anti-rabbit or goat anti-mouse) diluted to 5 μg / mL, added to each well, 200 μL / well, incubated in a 37°C incubator for 1 hour, then discarded the reaction solution; washed 4 times with washing solution (1*PBST); each...
Embodiment 3A
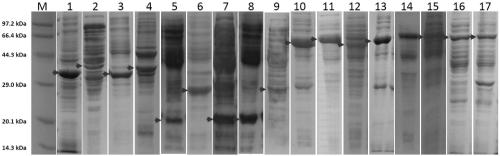
[0203] The DNA fragmentation activity comparison of embodiment 3ABP-L-DFE-1 (GsLDNase I) and ABP-L-DFE-3 (AsLDNase I)
[0204] Take 5-10ng equimolar concentration of the protein to be tested and 500ng BL21 genomic DNA, mix well in Buffer A (20mM Tris-HCl, 10mM Ca2Cl, pH 8.0), incubate at 37°C for 10min, and immediately run the sample on 1% agarose gel Electrophoresis to verify the effect of DNA fragmentation. Depend on Image 6 It can be seen that the DNA fragmentation activity of the same DFE fused with different ABPs is equivalent. Specifically, the DNA fragmentation activity of DNase I fused with the Protein A part and the Protein G part is equivalent at equimolar concentrations.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com