Use of cannabidiol in the preparation of anti-influenza medicine
A technology of cannabidiol and use, which is applied in the field of cannabidiol in the preparation of anti-influenza drugs, can solve the problems of high patient mortality and achieve the effect of inhibiting influenza virus
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
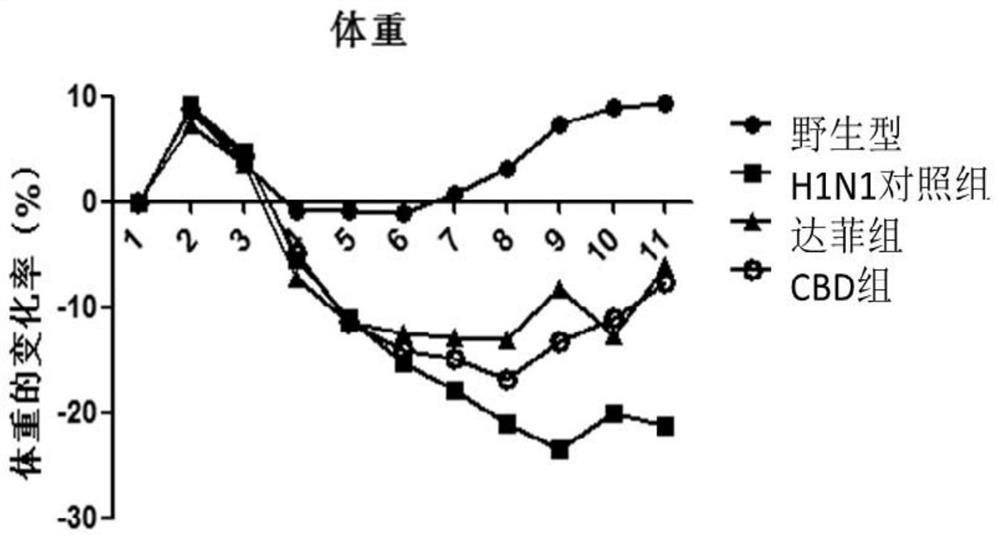
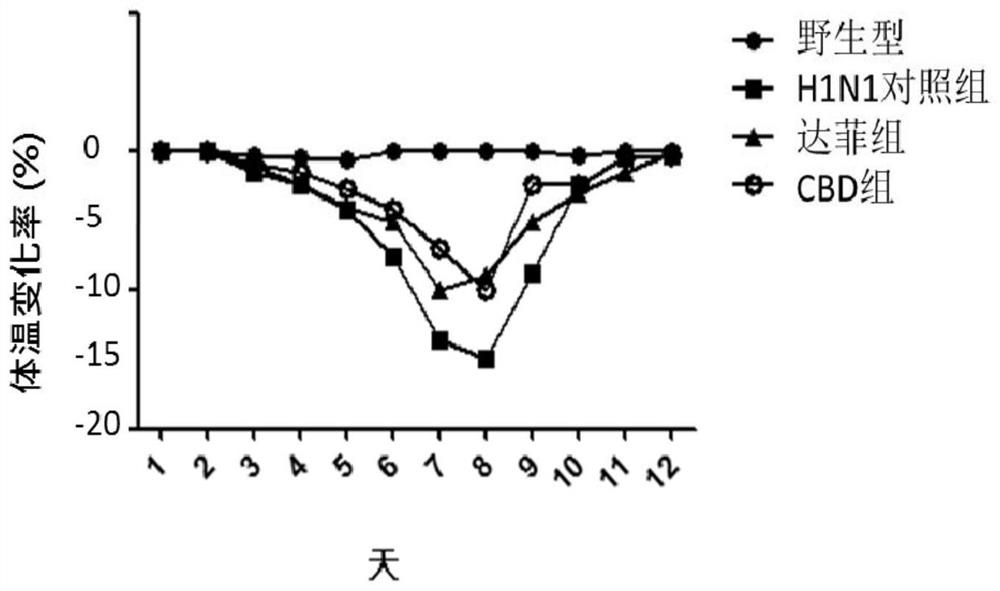
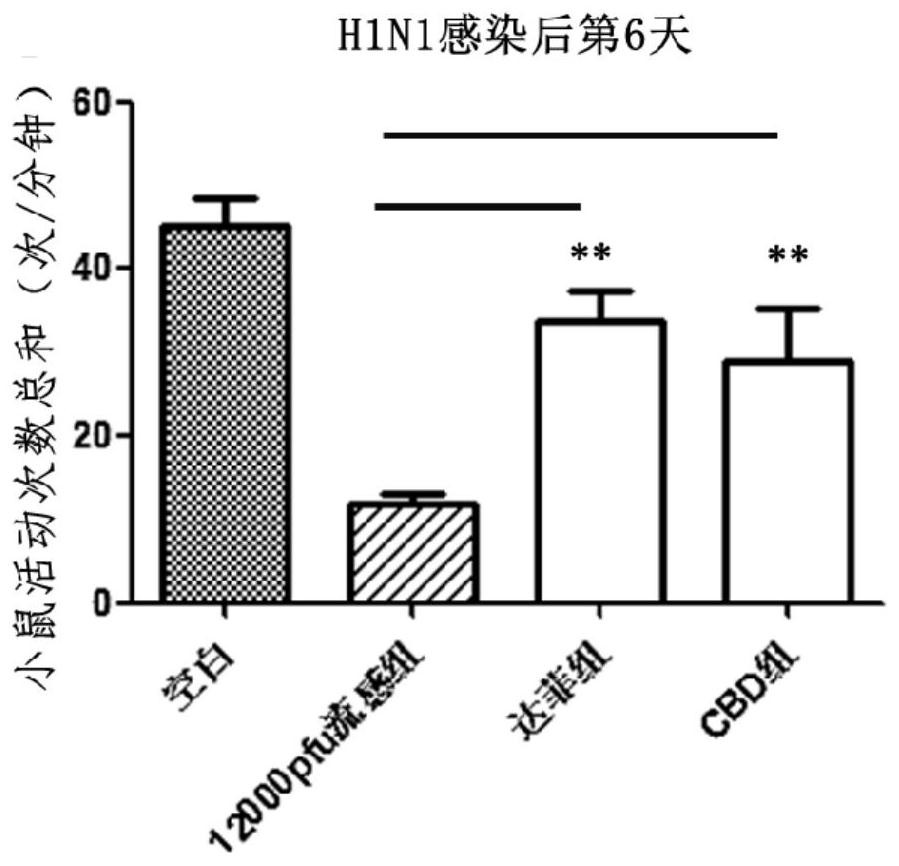
[0120] Embodiment 1: Animal experiment of cannabidiol anti-influenza A virus H1N1
[0121] 1. Experimental Animals and Preliminary Preparations
[0122] Six-week-old Kunming white male mice were purchased from Beijing Weitong Lihua Experimental Technology Co., Ltd.
[0123] Animals were routinely reared in the P2 and P3 animal centers (Biosafety level2 and 3, P2 / P3) of the Institute of Microbiology, Chinese Academy of Sciences. The photoperiod was 12 hours of light / 12 hours of darkness, and the animals had free access to food and water. Animals were adapted to the new environment for 1 day before relevant experiments. Animal experiments were approved by China Agricultural University and the Animal Ethics Committee of the State Key Laboratory of Agricultural Biotechnology (SKLAB-2017-3-002).
[0124] 9-11 day-old SPF chicken embryos were purchased from Beijing Meriyawei Experimental Animal Technology Co., Ltd.
[0125] 2. Experimental method
[0126] (1) Chicken embryo am...
Embodiment 2
[0203] Example 2: In vitro experiment of cannabidiol inhibiting RNA polymerase of influenza A virus H1N1
[0204] The passaged mouse bone marrow derived macrophages (bone-marrow derived macrophage, BMDM) and human non-small cell lung cancer cell line A549 ( CCL-185 TM ), inoculated in 6-well plate, and divided into the following 4 groups:
[0205] Control group (high glucose medium DMEM, the manufacturer is Sigma, the product number is D5648-1L, diluted to 1 liter with purified water before the experiment),
[0206] H1N1 infected control group,
[0207] CBD group (5μM) and
[0208] Tamiflu group (10 μM).
[0209] The above 4 groups were cultured overnight at 37°C, and the cells were infected with H1N1WSN when the confluence reached 100% and there was no gap between the cells within 18-24 hours. Cells were infected for 1 h at MOI=0.01.
[0210] The supernatant was aspirated and discarded, and the same volume of serum-free medium containing PBS, Tamiflu and CBD was adde...
Embodiment 3
[0223] Embodiment 3: The experiment of cannabidiol anti-influenza A virus H5N1
[0224] 1. Experimental Animals and Preliminary Preparation
[0225] 6-week-old C57BL / 6 male mice were purchased from Beijing Weitong Lihua Experimental Technology Co., Ltd.
[0226] Consistent with the conditions of Example 1. Animals were routinely reared in P2 and P3 Animal Centers (Biosafety level 2 and 3, P2 / P3) of the Institute of Microbiology, Chinese Academy of Sciences. The photoperiod was 12 hours of light / 12 hours of darkness, and the animals had free access to food and water. Animals were adapted to the new environment for 1 day before relevant experiments. Animal experiments were approved by China Agricultural University and the Animal Ethics Committee of the State Key Laboratory of Agricultural Biotechnology (SKLAB-2017-3-002).
[0227] C57BL / 6 male mice were randomly divided into 2 groups, with 10 mice in each group. From the 4th day of infection, intraperitoneal injection was adm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com