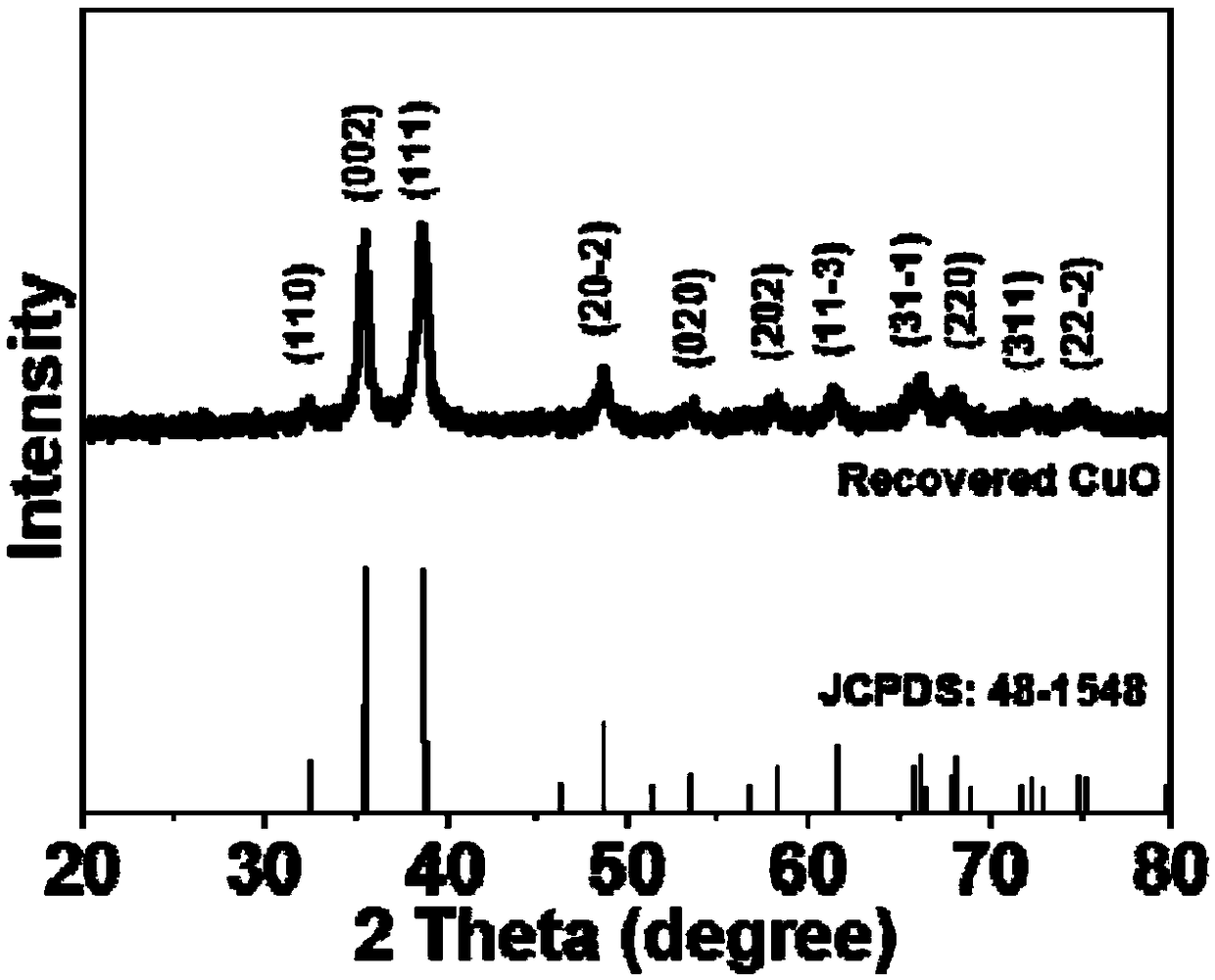
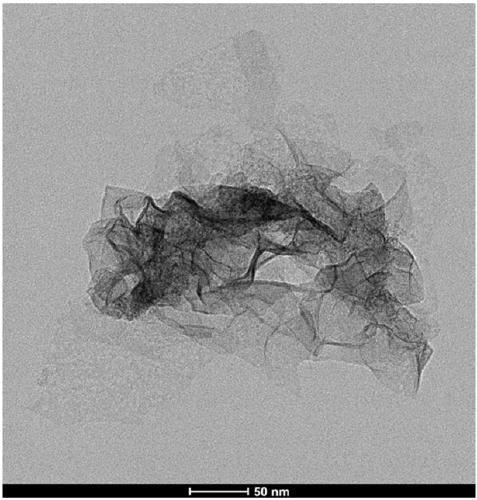
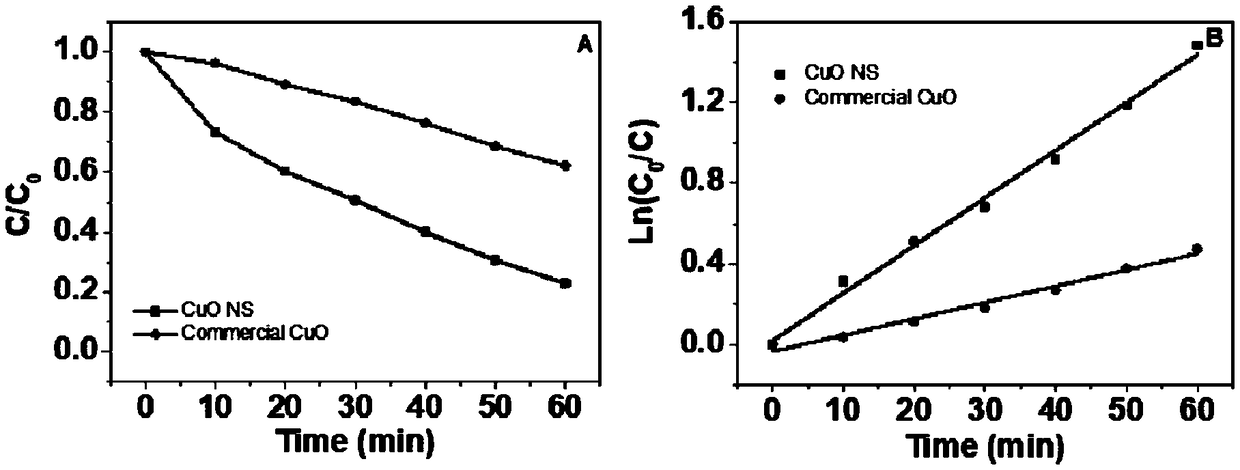
Method for greenly and efficiently recovering copper ions
A high-efficiency technology for copper recovery, applied in copper oxide/copper hydroxide and other directions, can solve the problems of high processing operation requirements, difficult equipment maintenance, low copper recovery rate, etc., achieve high-efficiency catalytic effect, reduce processing procedures, and reduce recovery costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Adjust the pH of 60mL 40mg / L copper sulfate solution to 5 and add 100mmol / L H 2 O 2 After stirring for 10 min, the obtained solid was filtered, washed and dried, and the residual copper ion concentration in the filtrate was measured, and the copper recovery rate was 49%.
[0034] Adjust 60mL of 40mg / L copper sulfate solution to pH 7, add 100mmol / L H 2 O 2 After stirring for 10 min, the obtained solid was filtered, washed and dried, and the residual copper ion concentration in the filtrate was measured to obtain a copper recovery rate of 50%.
[0035] Add 60mL 40mg / L copper sulfate solution, adjust the pH to 9, add 100mmol / L H 2 O 2 After stirring for 10 min, the obtained solid was filtered, washed and dried, and the residual copper ion concentration in the filtrate was measured to obtain a copper recovery rate of 50%.
[0036] Add 60mL 40mg / L copper sulfate solution, adjust the pH to 11, add 100mmol / L H 2 O 2 After stirring for 10 minutes, the obtained solid was filtered, washe...
Embodiment 2
[0039] Add 60mL 40mg / L copper sulfate solution, adjust the pH to 12, add 200mmol / L H 2 O 2 After stirring for 10 minutes, the obtained solid was filtered, washed and dried, and the residual copper ion concentration in the filtrate was measured to obtain a copper recovery rate of 99%.
[0040] Add 60mL 40mg / L copper sulfate solution, adjust the pH to 12, add 100mmol / L H 2 O 2 After stirring for 10 minutes, the obtained solid was filtered, washed and dried, and the residual copper ion concentration in the filtrate was measured to obtain a copper recovery rate of 99%.
[0041] Adjust the pH to 12 with 60mL 40mg / L copper sulfate solution, add 50mmol / L H 2 O 2 After stirring for 10 minutes, the obtained solid was filtered, washed and dried, and the residual copper ion concentration in the filtrate was measured to obtain a copper recovery rate of 99%.
[0042] Adjust 60mL 40mg / L copper sulfate solution to pH 12, add 25mmol / L H 2 O 2 After stirring for 10 minutes, the obtained solid was filt...
Embodiment 3
[0044] Add 60mL 40mg / L copper chloride solution, adjust the pH to 12, add 25mmol / L H 2 O 2 After stirring for 10 minutes, the obtained solid was filtered, washed and dried, and the residual copper ion concentration in the filtrate was measured to obtain a copper recovery rate of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com