Preparation method of fluorinated polyamide amine and application thereof serving as vaccine immune adjuvant
A technology of amidoamine and vaccine, which is applied in the field of biomedical materials, can solve problems such as hyperbranched polymodification, and achieve the effects of promoting intracellular release, promoting cytoplasmic delivery, and promoting cytoplasmic delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
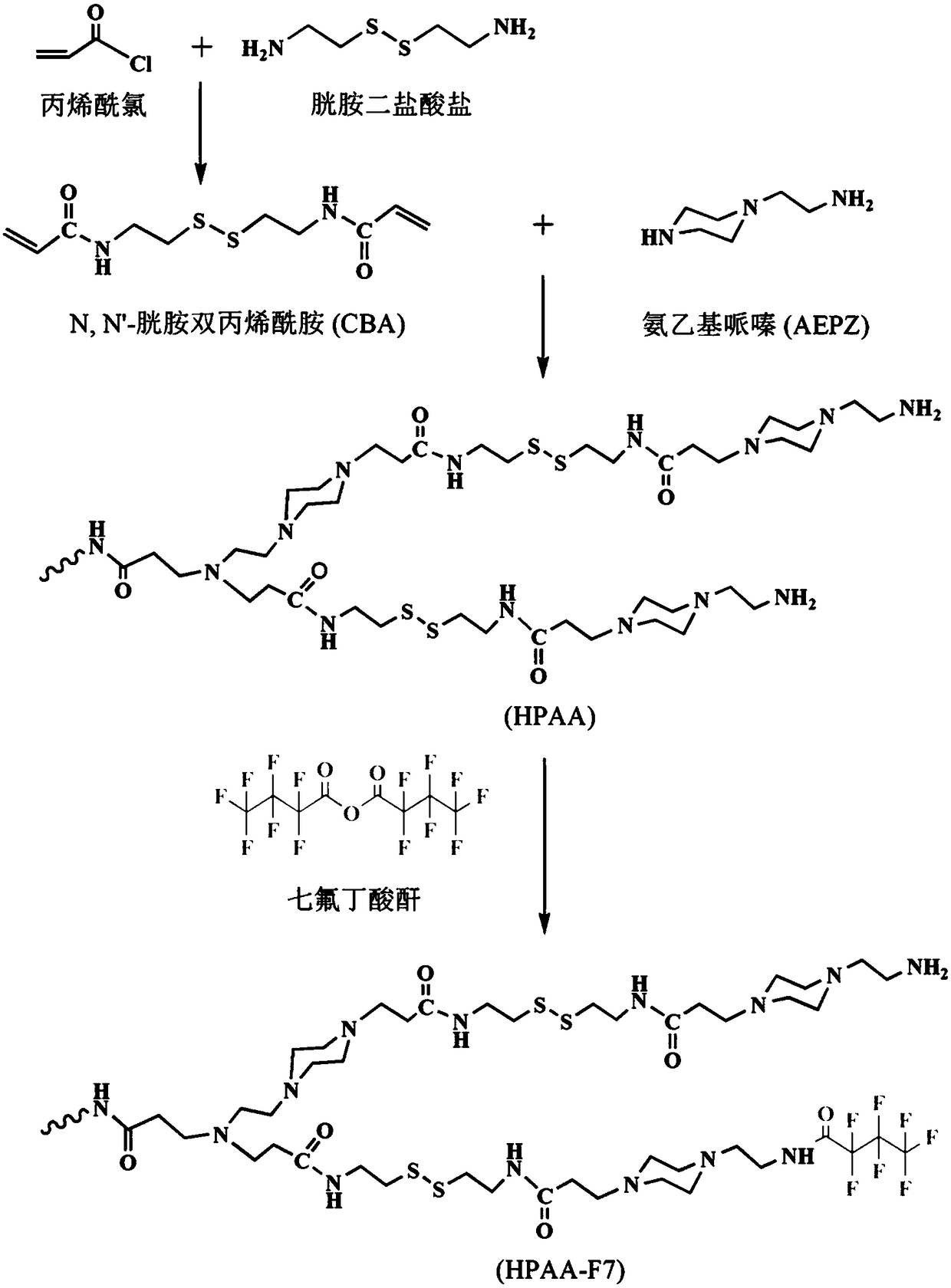
[0060] The synthetic route of fluorinated poly(amide amine) is as figure 1 shown. Made by the following steps:
[0061] (1) Synthesis of N,N'-cystamine bisacrylamide (CBA)
[0062] Use acryloyl chloride and cystamine dihydrochloride to synthesize the intermediate product N,N'-cystamine bisacrylamide (CBA). The specific steps are: dissolve 3.304mL acryloyl chloride (0.04mol) in 3.33mL dichloromethane (DCM) and NaOH solution (0.4 g / mL, 0.04 mol) was added dropwise to cystamine dihydrochloride solution (0.225 g / mL, 0.016 mol) alternately in an ice bath, and then the mixture was stirred at room temperature for 8 Hour. Then use 10 times the volume of DCM in the reaction mixture to extract the product in a separatory funnel, collect the lower layer extract, and wash with saturated NaHCO 3 solution, saturated NaCl solution and ultrapure water to wash the extract. The CBA was then collected during the process, the DCM was removed by a low pressure rotary evaporator and stored for...
Embodiment 2
[0070] The synthetic route of fluorinated poly(amide amine) is as figure 1 shown. Made by the following steps:
[0071] (1) Synthesis of N,N'-cystamine bisacrylamide (CBA)
[0072] The method is the same as step (1) in Example 1.
[0073] (2) Synthesis of poly(amide amine) (HPAA)
[0074] Method is the same as step (2) in embodiment 1.
[0075] (3) Synthesis of fluorinated poly(amide amine) (HPAA-F7)
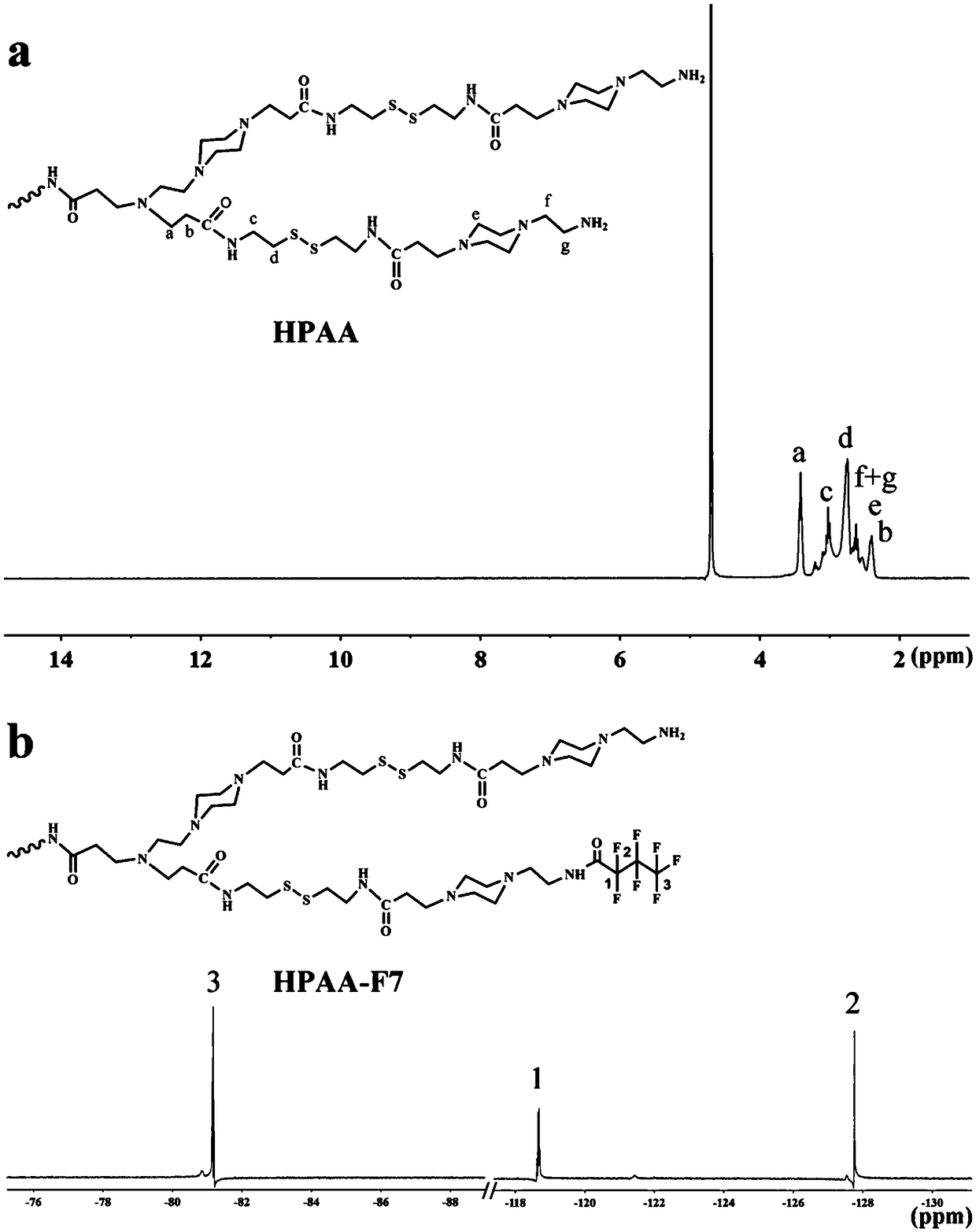
[0076] HPAA-F7 is synthesized by anhydride reaction, the specific steps are: 0.05g HPAA and 0.05g heptafluorobutyric anhydride (1.8mmol) are dissolved in 10mL methanol to obtain a methanol solution; then 0.03mL triethylamine (0.21mmol) Added directly to the methanol solution. The reaction mixture was stirred for 48 h and then dialyzed for 48 h against a dialysis membrane with a molecular weight cut off of 1000 using ultrapure water as the dialysate. The product was lyophilized to yield fluorinated poly(amidoamine) (HPAA-F7).
[0077] HPAA-F7 in D 2 in O 1 The F NMR col...
Embodiment 3
[0079] The synthetic route of fluorinated poly(amide amine) is as figure 1 shown. Made by the following steps:
[0080] (1) Synthesis of N,N'-cystamine bisacrylamide (CBA)
[0081] The method is the same as step (1) in Example 1.
[0082] (2) Synthesis of poly(amide amine) (HPAA)
[0083] Aqueous calcium chloride solution (0.04 g / mL, 13.2 mL) was mixed with CBA (0.64 g) dissolved in calcium chloride-methanol solution (0.04 g / mL, 40 mL). Then while the above solution was heated to 50°C, AEPZ (aminoethylpiperazine) (0.32 mL, 2.45 mmol) was added dropwise to the solution. After 24h, AEPZ (0.345mL, 2.64mmol) was added and reacted for 8h. After the reaction, the pH of the reaction mixture was adjusted to 4-6 with HCl solution (4 mmol / ml). Then ultrapure water was used as dialysate, dialyzed in a dialysis membrane with a molecular weight cut-off of 1000 for 48 hours, and then freeze-dried to obtain HPAA.
[0084] (3) Synthesis of fluorinated poly(amide amine) (HPAA-F7)
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com