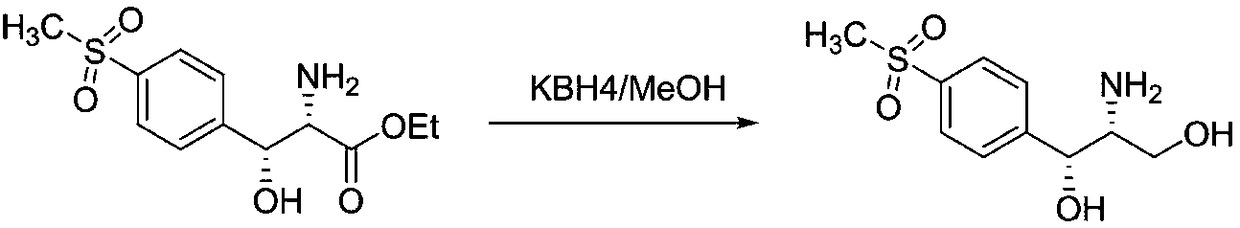
Preparation method for florfenicol intermediate, namely thiamphenicol amine
The technology of methylsulfonamide and methylsulfonyl phenylserine ethyl ester is applied in the field of synthesis process amplification of pharmaceutical intermediates, which can solve the problems of the material of the reaction kettle being flushed out of the kettle, the utilization rate of equipment is low, safety accidents and the like, and the reaction time can be shortened. , the effect of reducing the three wastes and improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
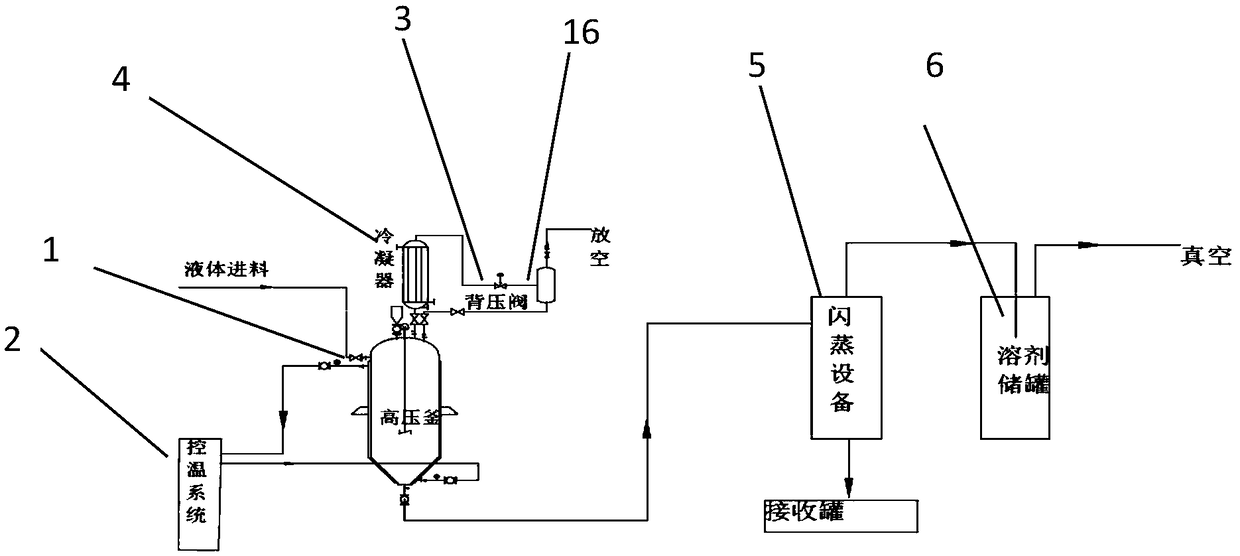
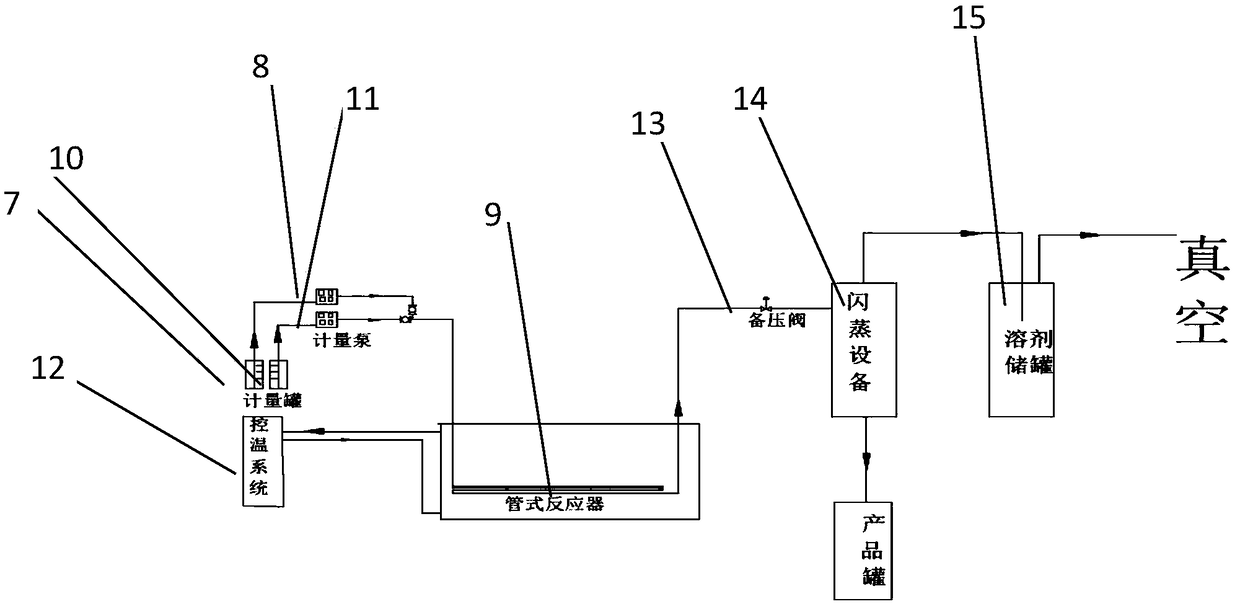
[0031] In the 1000L stainless steel reaction, first put 200 kg of methanol, use -15 °C frozen brine to control the temperature below 25 °C, put 30 kg of D-p-thymphenylphenylserine, use -15 °C frozen brine to control the temperature to about 25 °C, divide into 4 Add 7.2 kg of potassium borohydride in batches, and control the temperature not to exceed 40°C. After the reaction is stable, control the temperature at 45-50°C and keep warm for 2 hours. The reaction takes 8 hours from feeding to the end of the reaction. HPLC detects that thiamphenicolamine is 93.9%. By-product 4.5%, continue to prolong the heat preservation reaction time, the content no longer changes. After recovering the methanol, it enters into the cyclization reaction to generate 30.5 kg of cyclization product with HPLC purity: 96.7%.
Embodiment 2
[0033] In a 1000L stainless steel reactor, put 200 kg of methanol first, use -15°C frozen brine to control the temperature below 25°C, put 30 kg of D-p-thymphenylphenylserine, use -15°C frozen brine to control the temperature to about 25°C, divide Add 15 kg of potassium borohydride in 6 batches, control the temperature not to exceed 40°C, control the temperature at 45-50°C for 2 hours after the reaction is stable, and react for a total of 8 hours from feeding to the end of the reaction, monitoring 95.3% of thiamphenicolamine, methyl ester By-product 1.5%, continue to extend the heat preservation reaction time, the content no longer changes. After recovering the methanol, enter into the cyclization reaction to obtain 310 kg of cyclization product, HPLC purity: 98.6%.
[0034] As can be seen from the above examples, methyl esterification by-products always exist in the enlarged reaction scale, and there is no obvious improvement by prolonging the reaction time or increasing the ...
Embodiment 3
[0036] In a 1000L stainless steel reactor, put 200 kg of ethanol first, use -15 °C frozen brine to control the temperature below 25 °C, put 30 kg of D-p-thymphenylphenylserine, use -15 °C frozen brine to control the temperature to about 70 °C, divide Add 7.2 kg of potassium borohydride in 4 batches, and control the temperature not to exceed 80°C. After the reaction is stable, control the temperature at 70°C for 2 hours. The reaction will stop for a total of 10 hours from feeding to reaction. Continue to extend the heat preservation reaction time, and the content will not change. . After recovering ethanol, it enters the ring closure reaction. At the end of the reaction, 85.8% of the thiamphenicolamine was monitored, and 13% of the raw material remained. The cyclization reaction was carried out, and the yield was 24 kg. The HPLC purity was 89.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com