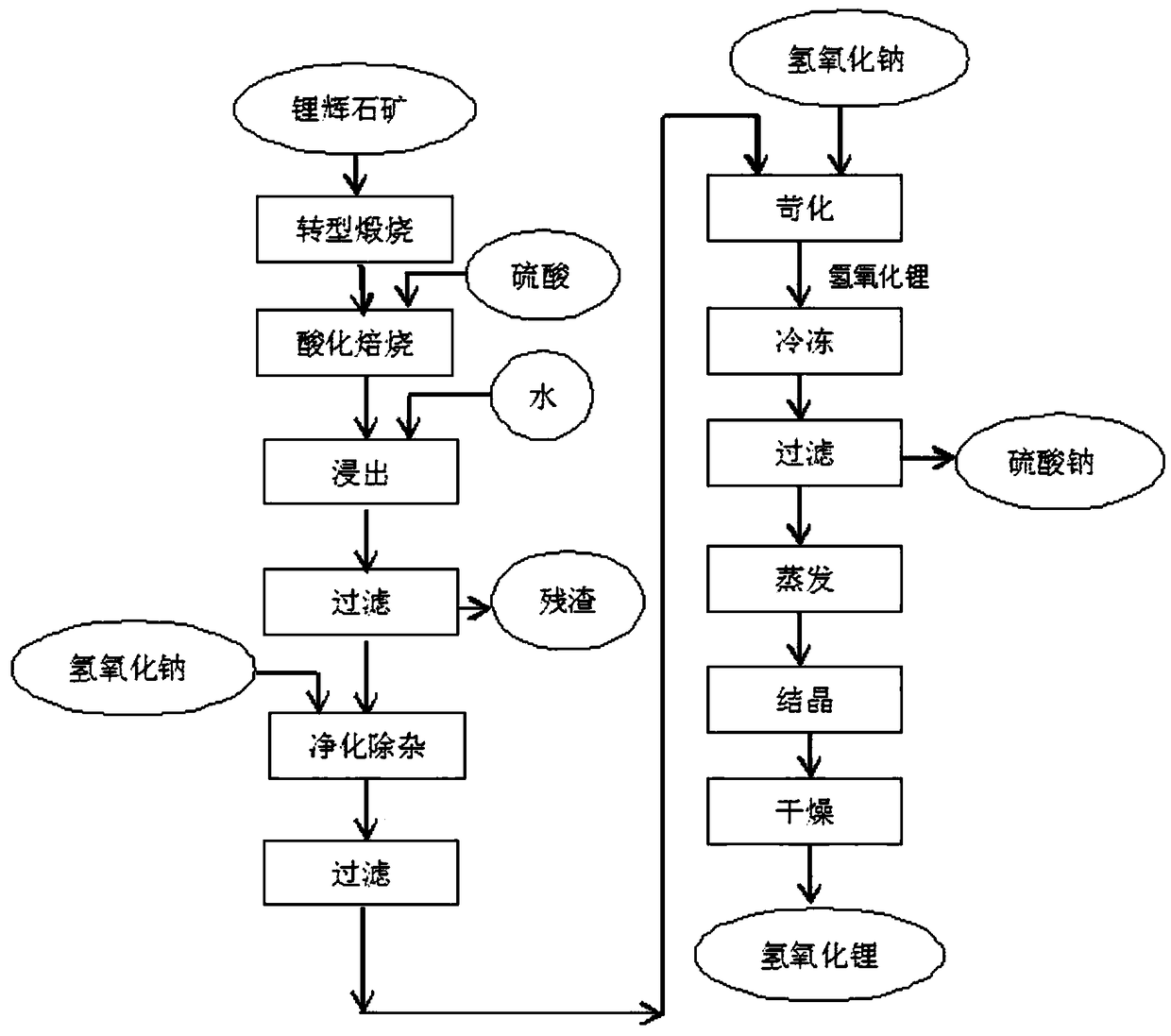
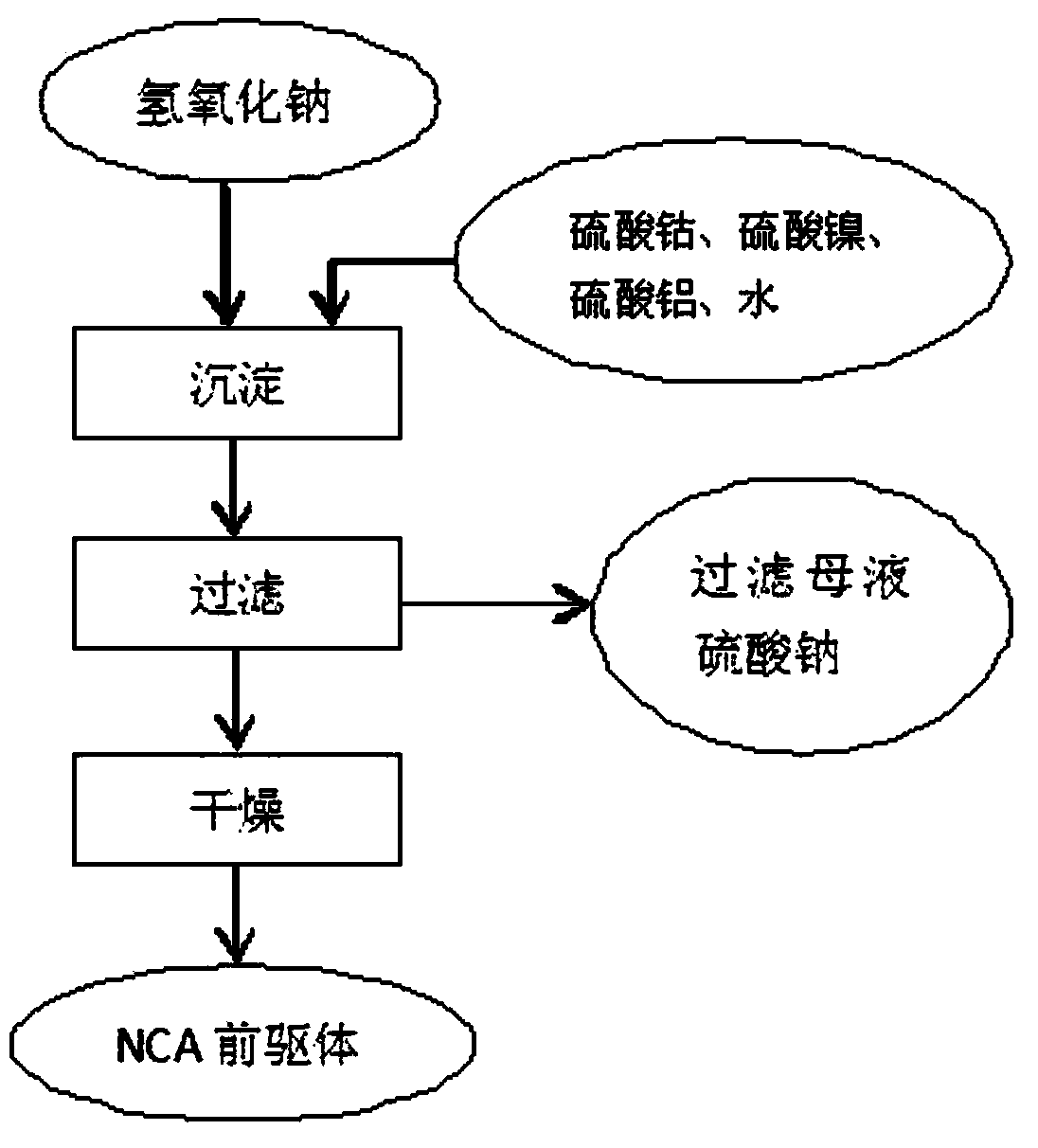
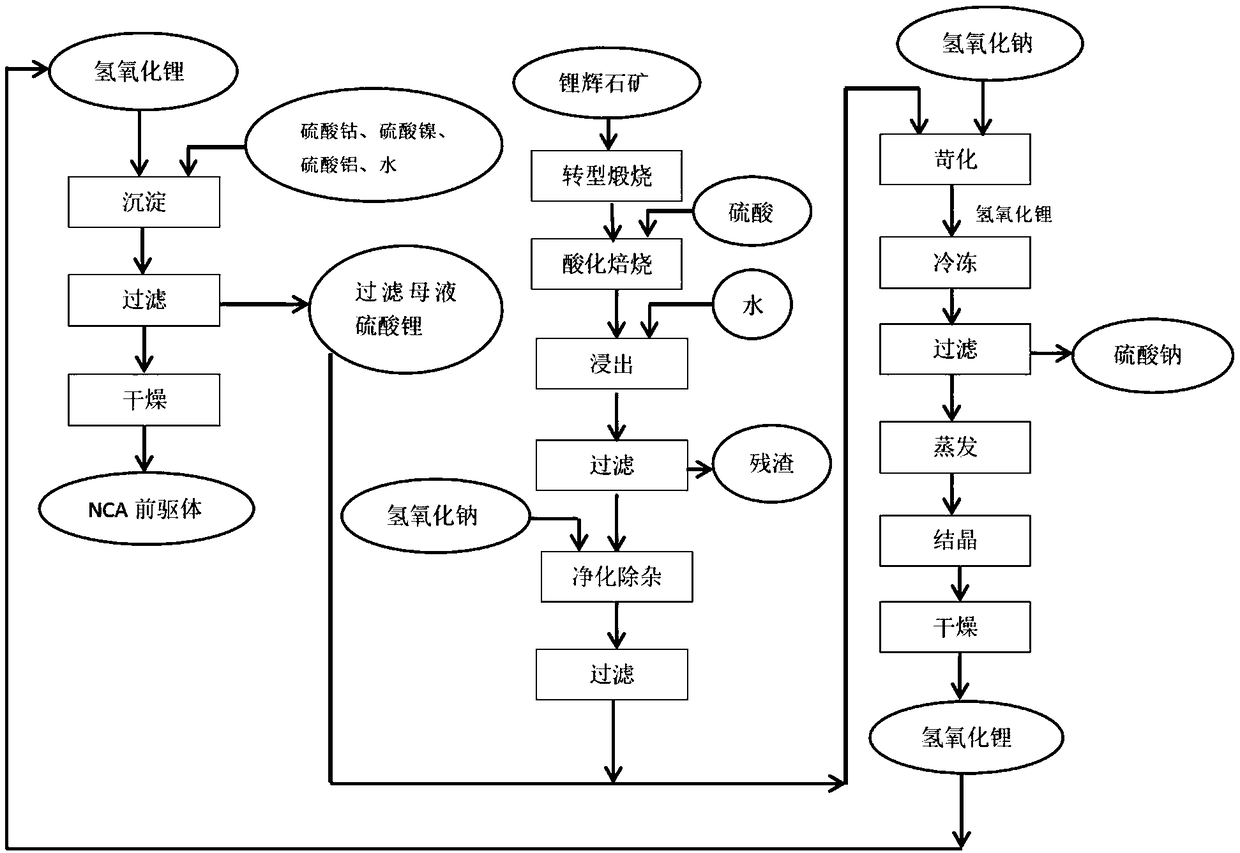
Method for fabricating NCA precursor by lithium circulation
A precursor, lithium sulfate technology, applied in the direction of electrical components, electrochemical generators, battery electrodes, etc., can solve problems such as heavy metal ion pollution, achieve the effect of improving reaction efficiency, reducing operating costs, optimizing morphology and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Take 462.6g of battery-grade nickel sulfate hexahydrate, 50.6g of cobalt sulfate heptahydrate, and 9.72g of aluminum sulfate octadecahydrate, add deionized water, and prepare a 1L solution, which is converted into the total The metal ion concentration is 2mol / l, this is solution A; take 167.8g of battery-grade lithium hydroxide monohydrate, add deionized water, and prepare a 1L solution, the converted lithium hydroxide concentration is 4mol / l, this is solution B . Use a 3L reactor to add solution B to solution A for co-precipitation reaction. The reaction conditions are: stirring intensity: medium, feeding time 30min, reaction time 2h, aging time 60min, reaction temperature 60°C. Vacuum filter while hot, save the filtrate, and return to the lithium hydroxide production system to prepare lithium hydroxide. Take out the filter cake, add 1L of deionized water, stir and wash at 60°C for 30 minutes, vacuum filter, save the filtrate, and use it to prepare lithium hydroxide s...
Embodiment 2
[0042] Take 462.6g of battery-grade nickel sulfate hexahydrate, 50.6g of cobalt sulfate heptahydrate, and 9.72g of aluminum sulfate octadecahydrate. The total metal ion concentration is 1.33mol / l, which is solution A; take 167.8g of battery-grade lithium hydroxide monohydrate, add deionized water, and prepare a 1L solution. The converted lithium hydroxide concentration is 4mol / l, which is Solution B. Use a 5L container as the reactor, add 400ml of deionized water as the bottom water, and continuously feed the two solutions of A and B in parallel flow, the feeding time is 30min, the reaction time is 4h, the aging time is 60min, and the reaction temperature is 90°C. Vacuum filter while hot, save the filtrate, and return to the lithium hydroxide production system to prepare lithium hydroxide. Take out the filter cake, add 1L of deionized water, stir and wash at 60°C for 30 minutes, vacuum filter, save the filtrate, and use it to prepare lithium hydroxide solution next time. Take ...
Embodiment 3
[0044] Take 462.6g of battery-grade nickel sulfate hexahydrate, 50.6g of cobalt sulfate heptahydrate, and 9.72g of aluminum sulfate octadecahydrate, add deionized water, and prepare a 1L solution, which is converted into the total The metal ion concentration is 2mol / l, which is solution A; take 167.8g of battery-grade lithium hydroxide monohydrate, add deionized water, and prepare a 1.5L solution, and the converted lithium hydroxide concentration is 2.67mol / l, which is Solution B. Use a 3L reactor to add solution A to solution B for co-precipitation reaction. The reaction conditions are: stirring intensity: medium, feeding time 30min, reaction time 8h, aging time 60min, reaction temperature 90°C. Vacuum filter while hot, save the filtrate, and return to the lithium hydroxide production system to prepare lithium hydroxide. Take out the filter cake, add 1L of deionized water, stir and wash at 60°C for 30 minutes, vacuum filter, save the filtrate, and use it to prepare lithium h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com