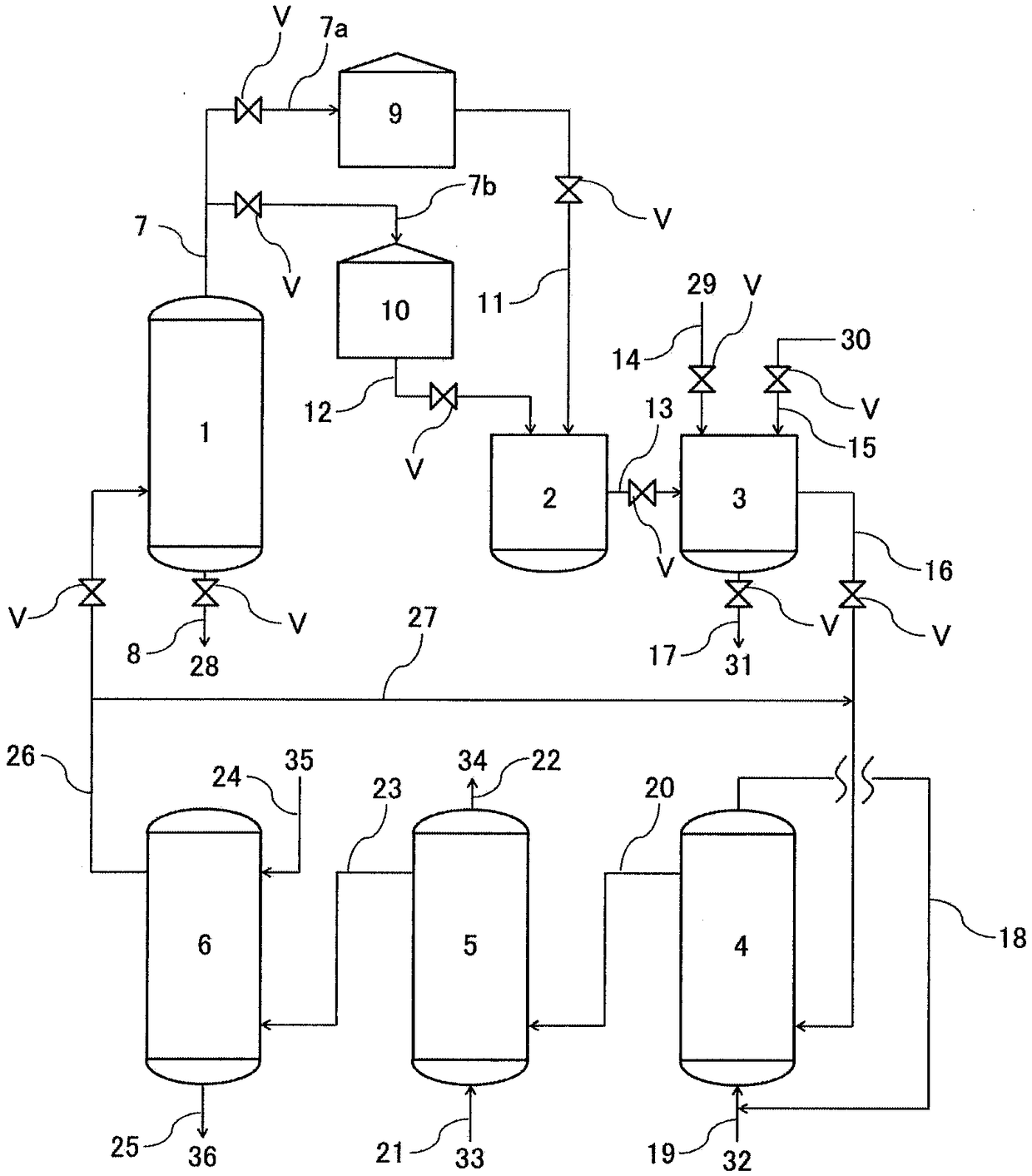
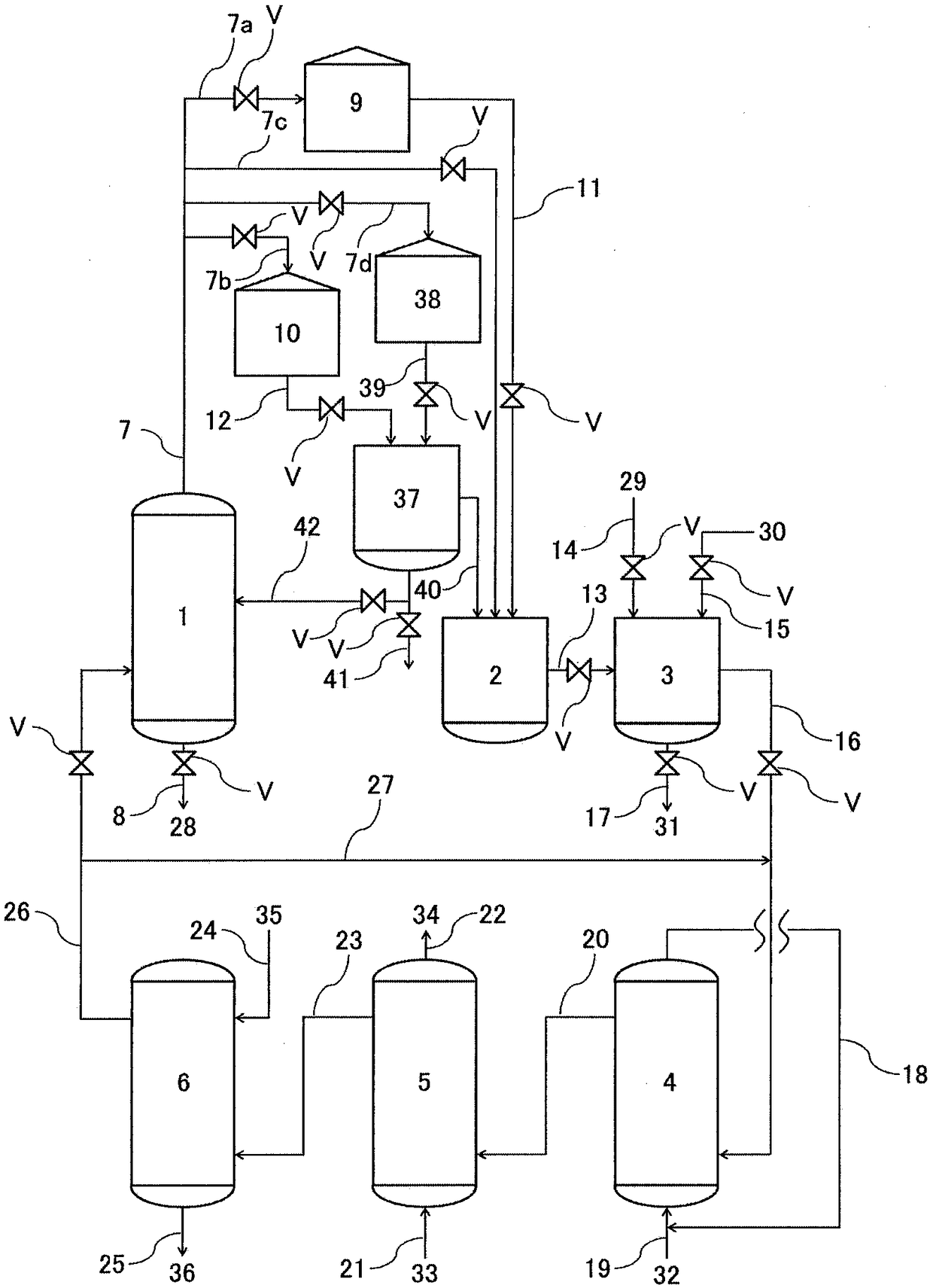
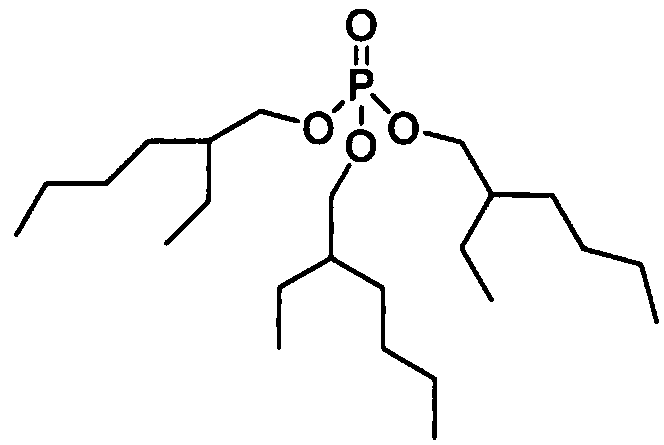
Method for producing hydrogen peroxide
A hydrogen peroxide and manufacturing method technology, applied in chemical instruments and methods, peroxide/peroxy hydrate/peroxyacid/superoxide/ozonide, inorganic chemistry, etc., can solve the problem of cycle process capacity decline and other issues, to achieve the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0141] The first distillation process and the second distillation process of the present invention were carried out on a small scale, and the initial working solution and the recycled working solution were compared.
[0142]
[0143] 400 g of the working solution was placed in a 500 mL flask provided in the distillation apparatus. Distillation is carried out under reduced pressure, and the vacuum degree is always controlled at 1.3kPa. The temperature in the flask was raised from room temperature to 182°C. The distillation was continued until finally no more distillate was distilled under the conditions of 1.3 kPa, 182°C.
[0144]
[0145]The residue obtained in the first distillation step is distilled at a pressure lower than that of the first distillation step. The degree of vacuum fluctuates between 0.03kPa and 0.15kPa within a period of time from the start of distillation, and finally stabilizes at 0.08kPa. The temperature in the flask was raised from room temperatur...
example 2
[0166] The first distillation process and the second distillation process of the present invention were implemented under different conditions from Example 1, and the initial working solution and the regenerated working solution for circulation were compared.
[0167]
[0168] 351 g of the working solution was placed in a 500 mL flask provided in the distillation apparatus. Distillation is carried out under reduced pressure, and the vacuum degree is always controlled at 1.3kPa. The temperature in the flask was raised from room temperature to 157°C. The distillation was continued until finally no more distillate was distilled under the conditions of 1.3 kPa, 157°C.
[0169]
[0170] The residue obtained in the first distillation step is distilled at a pressure lower than that of the first distillation step. The degree of vacuum fluctuates between 10Pa and 150Pa within a period of time from the start of distillation, and finally stabilizes at 0.01kPa to 0.04kPa. The tempe...
example 3
[0185] The first distillation process and the second distillation process of the present invention were implemented under different conditions from Examples 1 and 2, and the initial working solution and the regenerated working solution for circulation were compared.
[0186]
[0187] 351 g of the same working solution as in Example 2 was placed in a 500 mL flask included in the distillation apparatus. Distillation is carried out under reduced pressure, and the vacuum degree is always controlled at 1.3kPa. The temperature in the flask was raised from room temperature to 180°C. The distillation was continued until finally no more distillate was distilled under the conditions of 1.3 kPa, 180°C.
[0188]
[0189] The residue obtained in the first distillation step is distilled at a pressure lower than that of the first distillation step. The vacuum degree is always controlled at 0.13kPa. The temperature in the flask was raised from room temperature to 250°C. The distillati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com