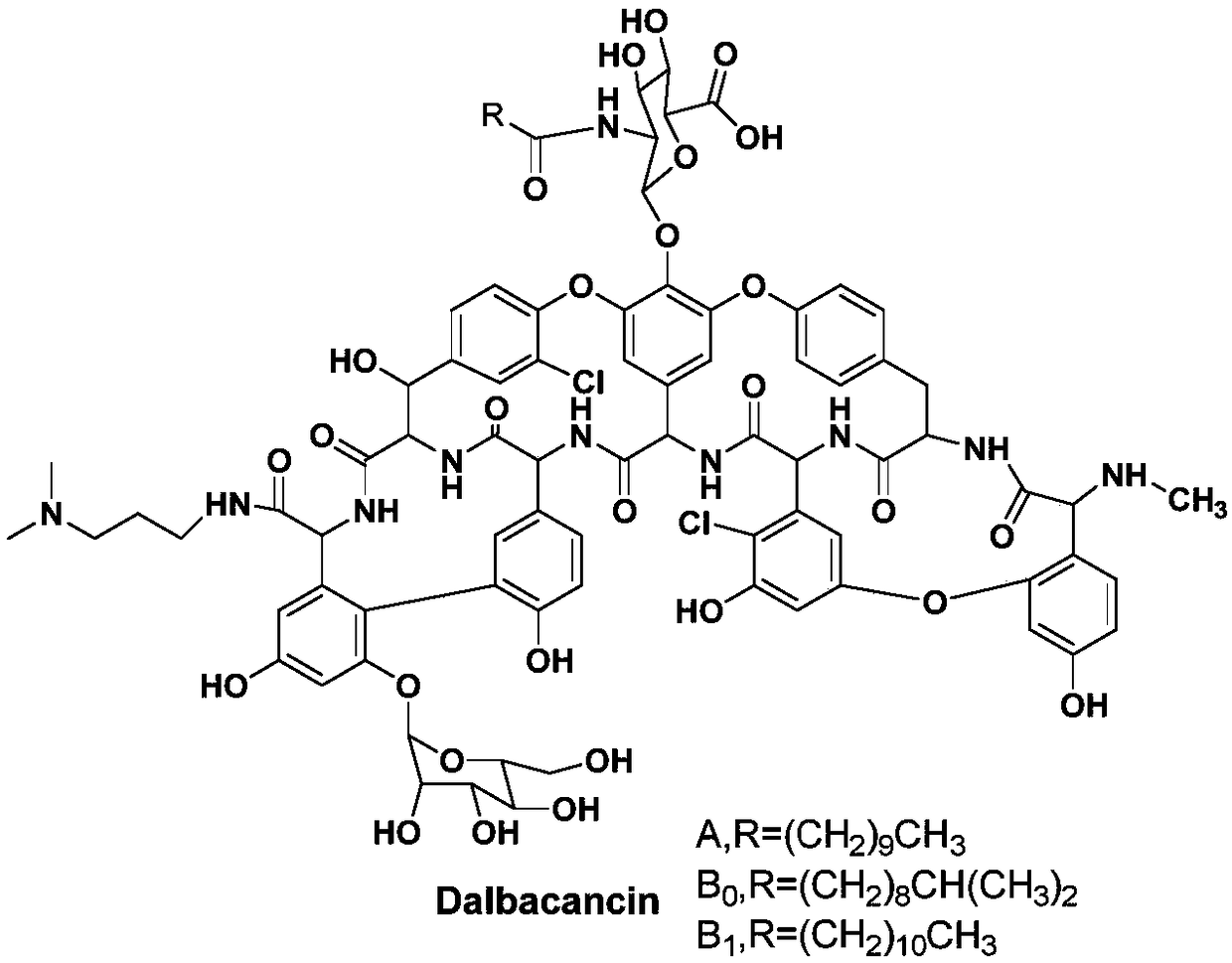
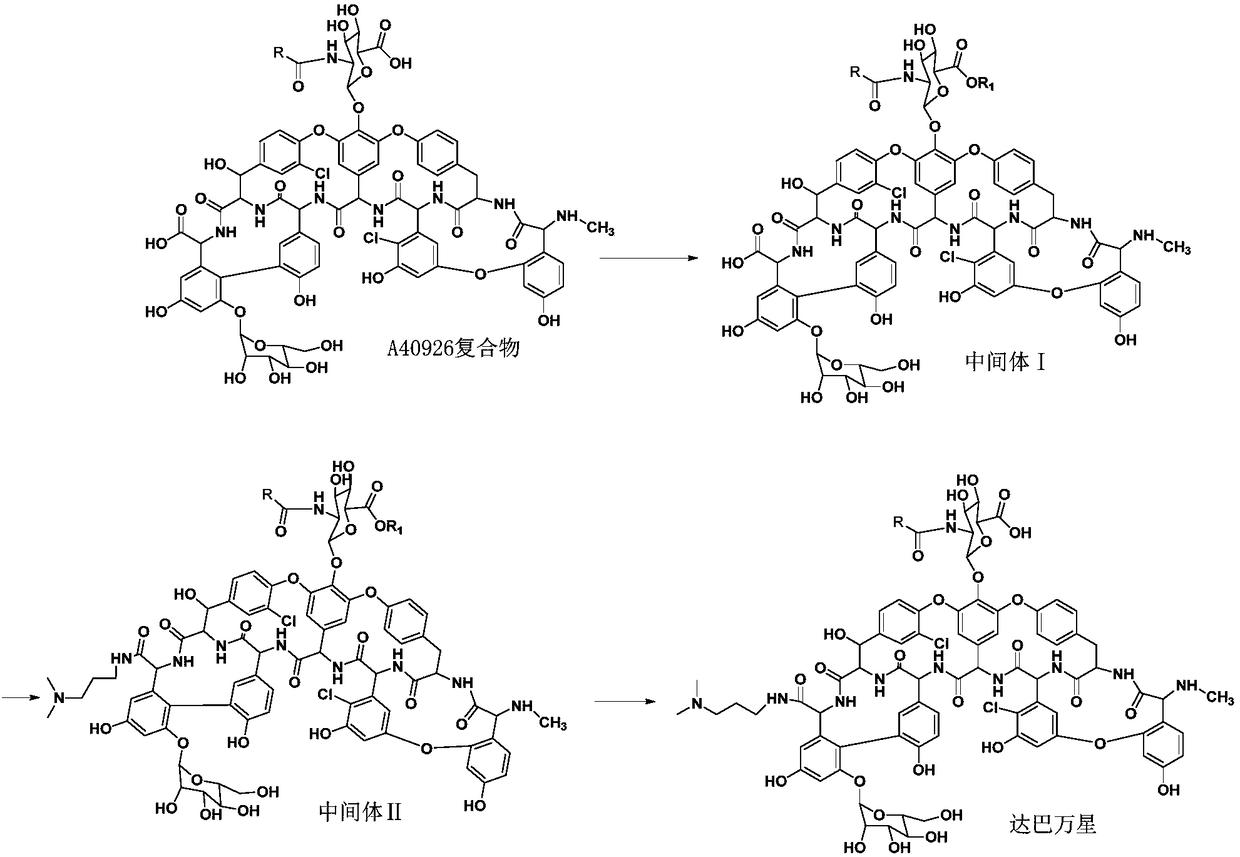
Method for preparing dalbavancin
A technology of dalbavancin and intermediates, which is applied in the field of antibiotic preparation, can solve the problems of difficulty in obtaining dalbavancin, high purification cost and the like, and achieves the effects of reduced preparation cost, easy removal and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A preparation method of dalbavancin, the synthetic route is as follows:
[0030]
[0031] In the formula, R 1 It is a C4-C30 hydrocarbon group;
[0032] Including the following steps:
[0033] 1) Protecting the carboxyl group of the A40926 complex to obtain intermediate I;
[0034] 2) Amidation reaction of intermediate I with 3,3-dimethylamino-1-propylamine to obtain intermediate II;
[0035] 3) The intermediate II is hydrolyzed in alkaline solution, acidified and purified to obtain dalbavancin.
[0036] As a further improvement of the above preparation method, using R 1 The carboxyl group of the A40926 complex was protected by the esterification reaction between -OH and the A40926 complex.
[0037] As a further improvement of the above preparation method, R 1 It is a C4-C30 chain alkyl group. Considering the cost of raw materials, reaction conditions, etc., C4-C20 alkanyl is preferred. Further, R 1 -OH is a hydroxyl-terminated alcohol.
[0038] As a furthe...
Embodiment 1
[0049] 1) Add 100ml of n-butanol to a three-necked flask, cool to 5°C, add concentrated hydrochloric acid dropwise, adjust the pH value to 1.0-1.5, add A40926 complex (10.00g, 5.77mmol), and stir the reaction at 3-5°C After 8 hours, triethylamine was slowly added dropwise to adjust the pH to 5.5 when precipitation appeared, filtered, and dried in vacuum below 35°C to obtain 9.60 g of intermediate I with a yield of 92%;
[0050] 2) Dissolve Intermediate I (9.50g, 5.28mmol) in 150ml DMSO at 25-30°C, add 3,3-dimethylamino-1-propanamine (0.65g, 6.36mmol) and DCC (1.30g , 6.35mmol), stirred and reacted at 20°C for 8 hours, added 170ml of methyl tert-butyl ether to precipitate, filtered, and vacuum-dried below 35°C to obtain 8.80g of intermediate II, with a yield of 84%;
[0051] 3) Dissolve intermediate II (8.70g, 4.83mmol) in a mixed solution of 10ml of acetonitrile and water, slowly add 1mol / L LiOH solution dropwise at 5°C to pH 10, and continue to stir and react at this temperat...
Embodiment 2
[0054] 1) Add 100ml of phenylethanol to a three-necked flask, cool to 5°C and add concentrated sulfuric acid dropwise to adjust the pH to 1.0-1.5. Add A40926 complex (10.00g, 5.77mmol), stir and react at 5-8°C for 10 hours, slowly add triethylamine dropwise to adjust the pH to 5.5 when precipitation occurs, filter, and vacuum dry below 35°C to obtain intermediate I9. 89g, the yield is 94%;
[0055] 2) 20~25°C, dissolve intermediate I (9.50g, 5.13mmol) in 170ml DMF, add 3,3-dimethylamino-1-propanamine (0.65g, 6.36mmol) and benzotrifluorophosphate Azol-1-yl-oxytripyrrolidinylphosphonium (3.40 g, 6.35 mmol). Stir the reaction at 30°C for 12 hours, add 170ml of methyl tert-butyl ether to precipitate, filter, and dry under vacuum below 35°C to obtain 8.28g of intermediate II with a yield of 83%;
[0056] 3) Take intermediate II (8.00g, 4.14mmol) and dissolve it in a mixed solution of 10ml tetrahydrofuran and water, slowly add 1mol / L NaOH solution dropwise at 5°C to pH 9, and cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com