Two-dimensional metalloporphyrin-based COF (chip on film) material as well as film preparation method and application
A porphyrin-based, metal-based technology, applied in chemical instruments and methods, water/sludge/sewage treatment, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of little research on photocatalytic performance, Achieve the effect of improved catalytic degradation performance, large specific surface area, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of CuP-DHNDA-COF:
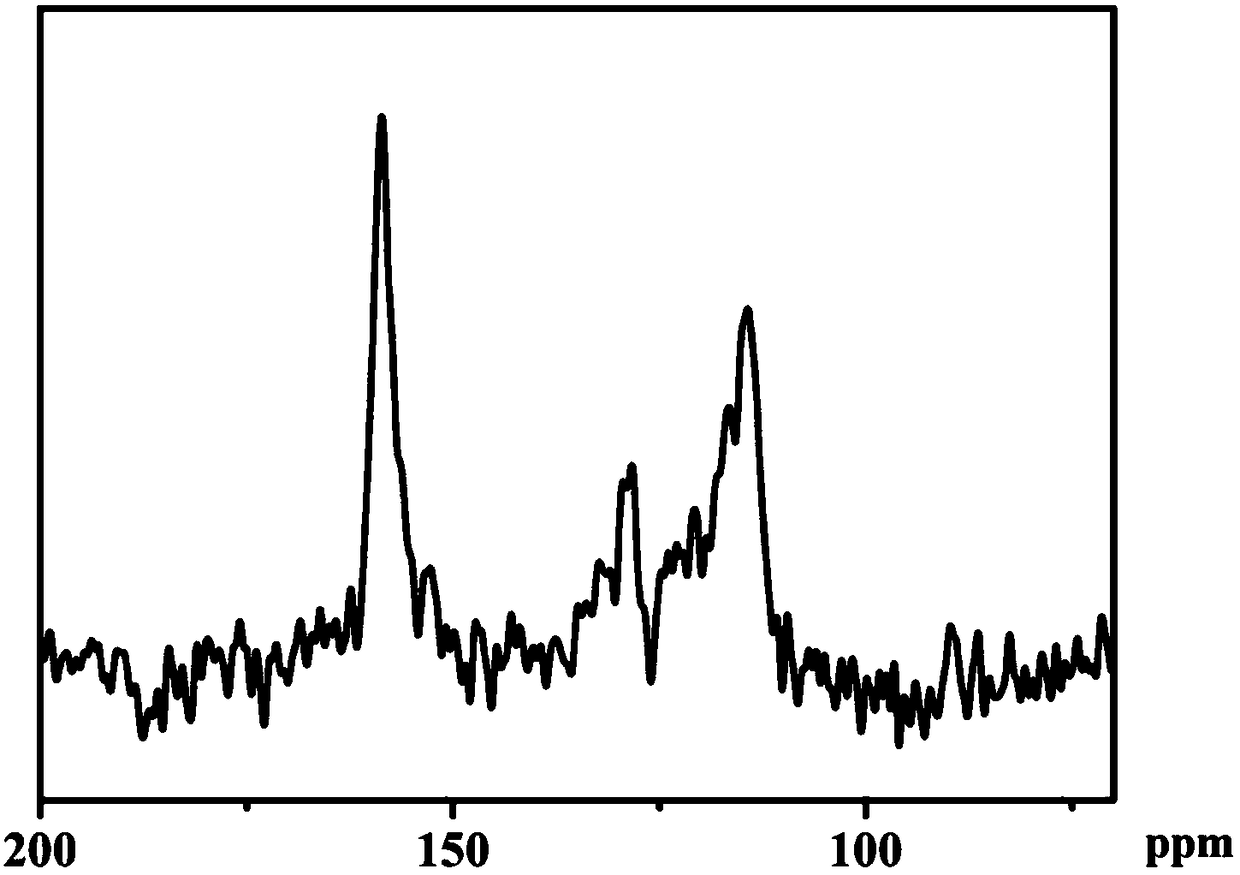
[0032] Copper 5,10,15,20-tetrakis-(4-aminophenyl)porphyrin (CuTAPP) (88.2mg, 0.12mmol), 2,6-dihydroxy-1,5-dialdehydenaphthalene (58.6mg ,0.24mmol), 0.6mL36% acetic acid, dichlorobenzene (3mL), and n-butanol (3mL) were added to a polytetrafluoroethylene-lined autoclave, and after ultrasonic dispersion for 20min, the autoclave was sealed and placed in an oven , 120 ° C reaction 3d. Then naturally cool to room temperature, centrifuge, and then wash with N,N-dimethylformamide, tetrahydrofuran, and acetone in order to wash away unreacted precursors and oligomers. Then vacuum-dried at 80° C. for 12 h to obtain 116.2 mg of a purple solid (theoretical yield: 138.2 mg), with a yield of 84.1%.
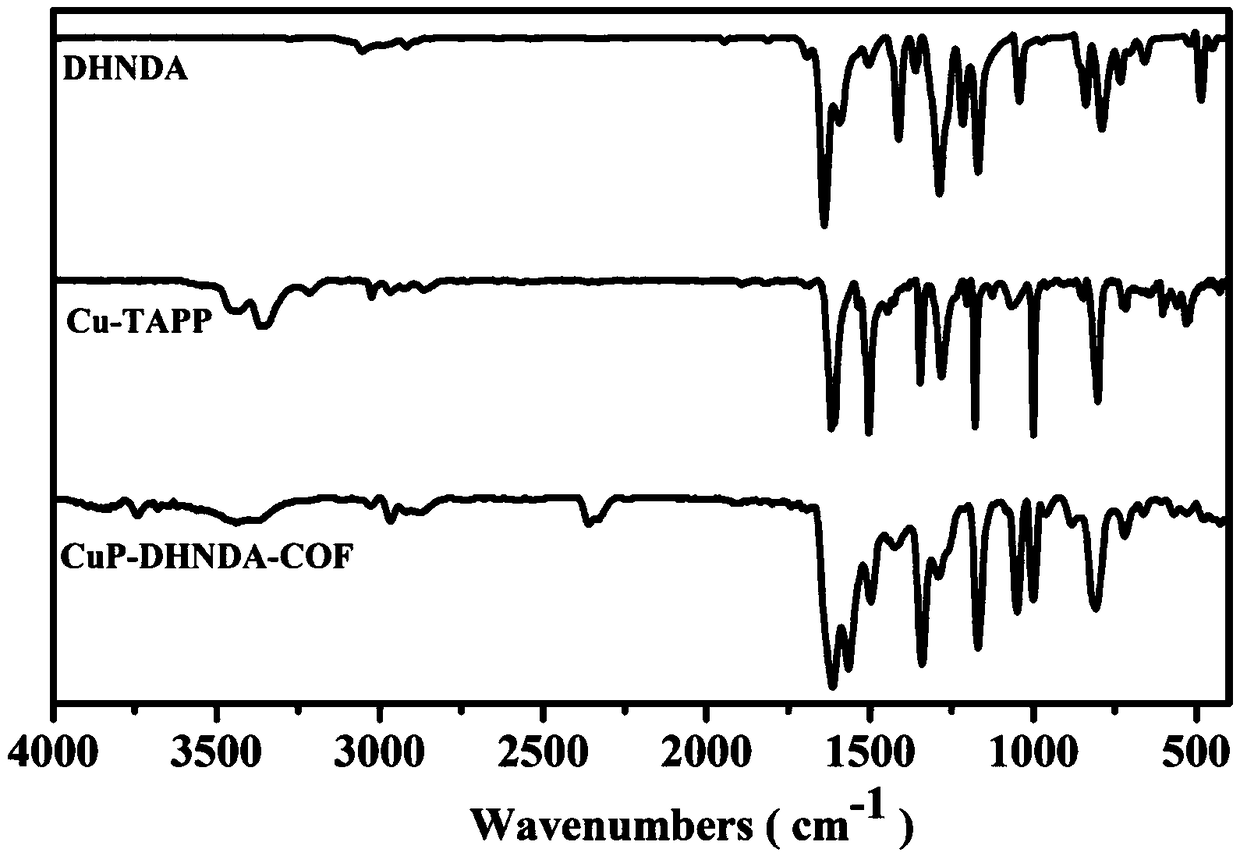
[0033] Such as figure 1 As shown, the product CuP-DHNDA-COF is at 1586cm -1 There is a strong absorption, which is the stretching vibration peak of the formed imine bond C=N, while the aldehyde group C=O in the raw material (1658cm -1 ) and amino N-H (32...
Embodiment 2
[0041] Photodegradation of methylene blue experiment: In order to further explore the performance of the synthesized CuP-DHNDA-COF material, a series of experiments were designed to verify the efficiency of its catalytic degradation of methylene blue.
[0042] Take 10 mg of the catalyst (CuP-DHNDA-COF), add 20 mg (10 mg / L) of methylene blue aqueous solution to it, and put the mixture into an ultrasonic cleaner to completely disperse the catalyst in the methylene blue aqueous solution. According to the above operation, prepare six test tubes for adding the above reaction solution, numbered 1, 2, 3, 4, 5, 6. After adding methylene blue aqueous solution and catalyst to No. React under dark conditions for 1 hour, and measure the UV-visible absorbance value of No. 2 after centrifugation. Measure the absorbance of No. 3 test tube after 15 minutes of visible light irradiation reaction, and measure the absorbance of the solutions in No. 4, No. 5 and No. 6 test tubes after 30 minutes, ...
Embodiment 3
[0045] Preparation of CuP-DHNDA-COF thin film: After amination treatment of ITO glass, first react with 2,6-dihydroxy-1,5-dialdehyde naphthalene, then take it out and wash it, put it in 5,10,15, 20-tetrakis-(4-aminophenyl)porphyrin copper solution followed by reaction, then removed and washed, and then put into the previous 2,6-dihydroxy-1,5-dialdehyde naphthalene solution, repeating this for 50 Take it out, wash it, dry it, and measure SEM to find that a dense film has formed.
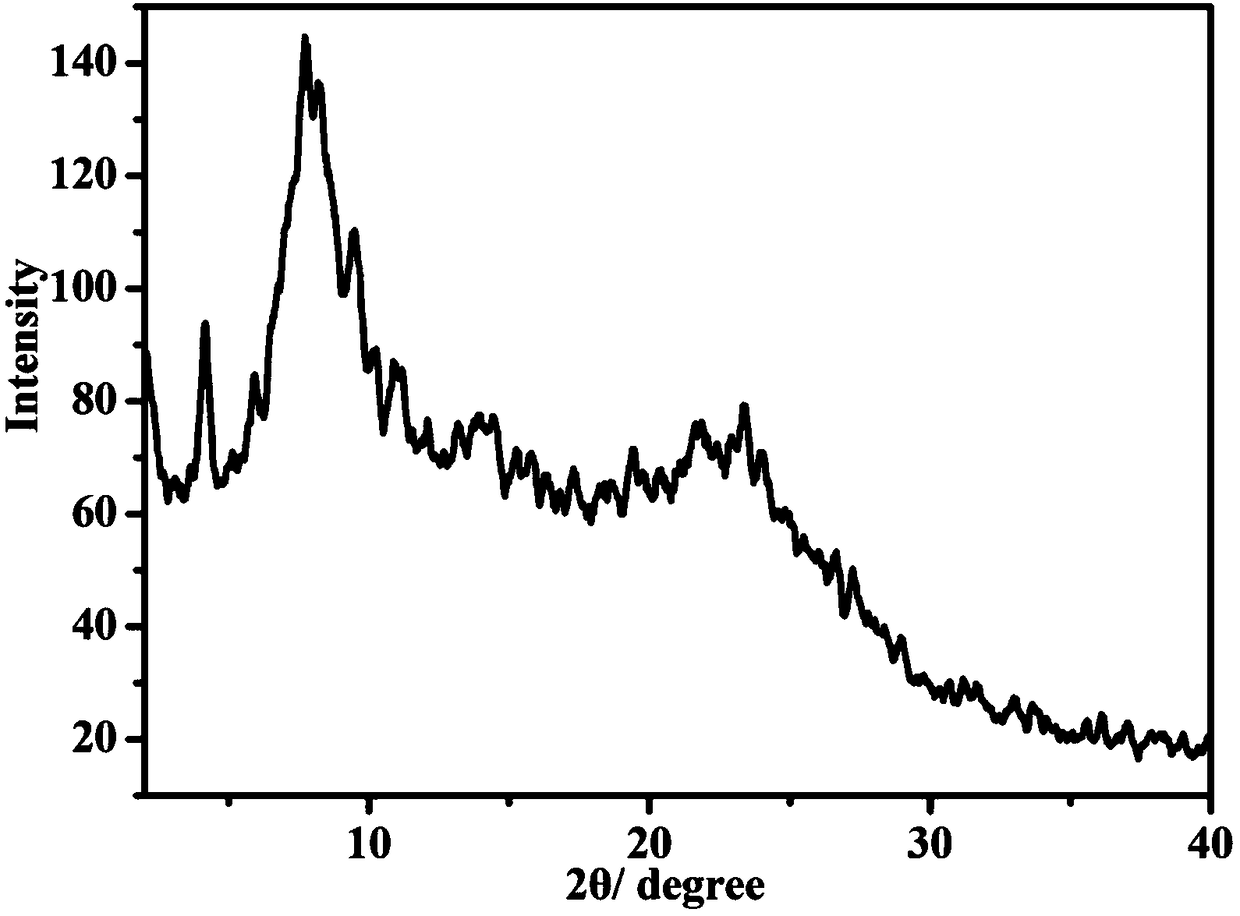
[0046] Such as Figure 9 As shown, the XRD of CuP-DHNDA-COF film and powder have similar shapes, and the peak positions are consistent, indicating that the synthesized CuP-DHNDA-COF film has the same framework structure as the powder.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com