Metal coordination porphyrin-based conjugated polymer as well as preparation method and application thereof in photocatalytic degradation of organic pollutants
A conjugated polymer, organic pollutant technology, applied in the direction of organic compound/hydride/coordination complex catalysts, water pollutants, physical/chemical process catalysts, etc., can solve the problems of aggravating metal ion pollution and high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The present invention at first synthesizes metal coordination porphyrin monomer, and concrete steps are as follows:
[0047] Into a 500 mL three-necked flask, 11 g of 4-nitrobenzaldehyde, 300 mL of propionic acid and 12 mL of acetic anhydride were sequentially added. Then, under a nitrogen atmosphere, heat up to 150 °C and reflux, then add 5 mL of pyrrole to it, and continue the reflux reaction for 30 min. After the reaction is naturally cooled to room temperature, the black precipitate is collected, washed with ultrapure water and methanol, and placed in a vacuum at 60 °C. Dry in the oven for 12 h. The obtained powder was dissolved in 35 mL of pyridine, refluxed for 60 min, cooled to 0 °C and placed in a refrigerator for 6 h. The precipitate was collected by filtration and washed with acetone until the filtrate was colorless to obtain a dark purple solid powder, which was dried in a vacuum oven at 60 °C for 12 h.
[0048] Dissolve 2.0 g of the above dark purple solid...
Embodiment 2
[0051] The specific steps for the synthesis of 4,7-bis(4-formylphenyl)-2,1,3-benzothiadiazole (BT) monomer are as follows:
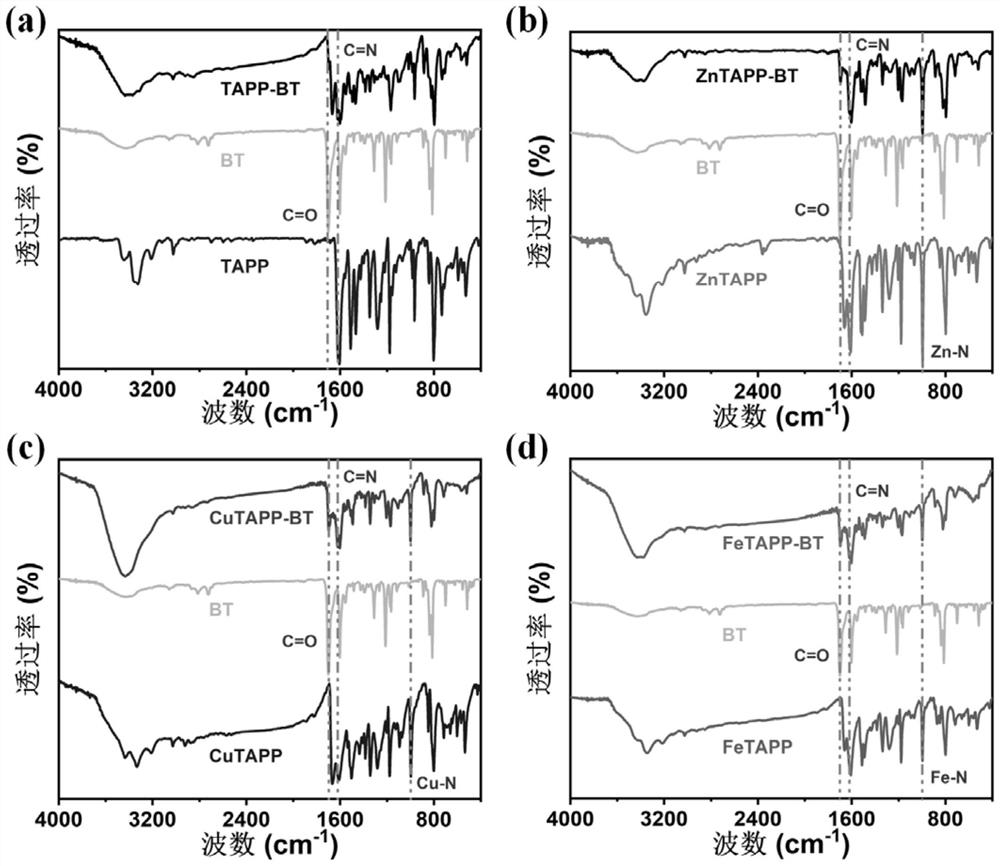
[0052] Add 9 mL of 6mol / L cesium carbonate aqueous solution to a 100 mL one-necked flask and wash with N 2 Degas for 20 min. Then, 25 mL of anhydrous toluene and 17 mL of absolute ethanol, 1.25 g of 4-formylphenylboronic acid, 1 g of 4,7-dibromo-2,1,3-benzothiadiazole and 200 mg of tetra( Triphenylphosphino) palladium, and degassed twice, 20 min each time. Reflux reaction at 75 °C for 12 h under a nitrogen atmosphere. After the reaction, the mixed system was poured into water and extracted three times with chloroform. The organic solvent was removed by a rotary evaporator to obtain a crude product. Finally, the obtained 4,7-bis(4-formylphenyl )-2,1,3-benzothiadiazole, dried in a vacuum oven at 60 °C. Product infrared spectrum can be seen, wherein 1701 cm -1 Corresponding to the aldehyde groups at both ends of the molecule, 1602cm -1 Corresponding t...
Embodiment 3
[0054] The preparation of porphyrin-based conjugated polymer (TAPP-BT), the specific steps are as follows:
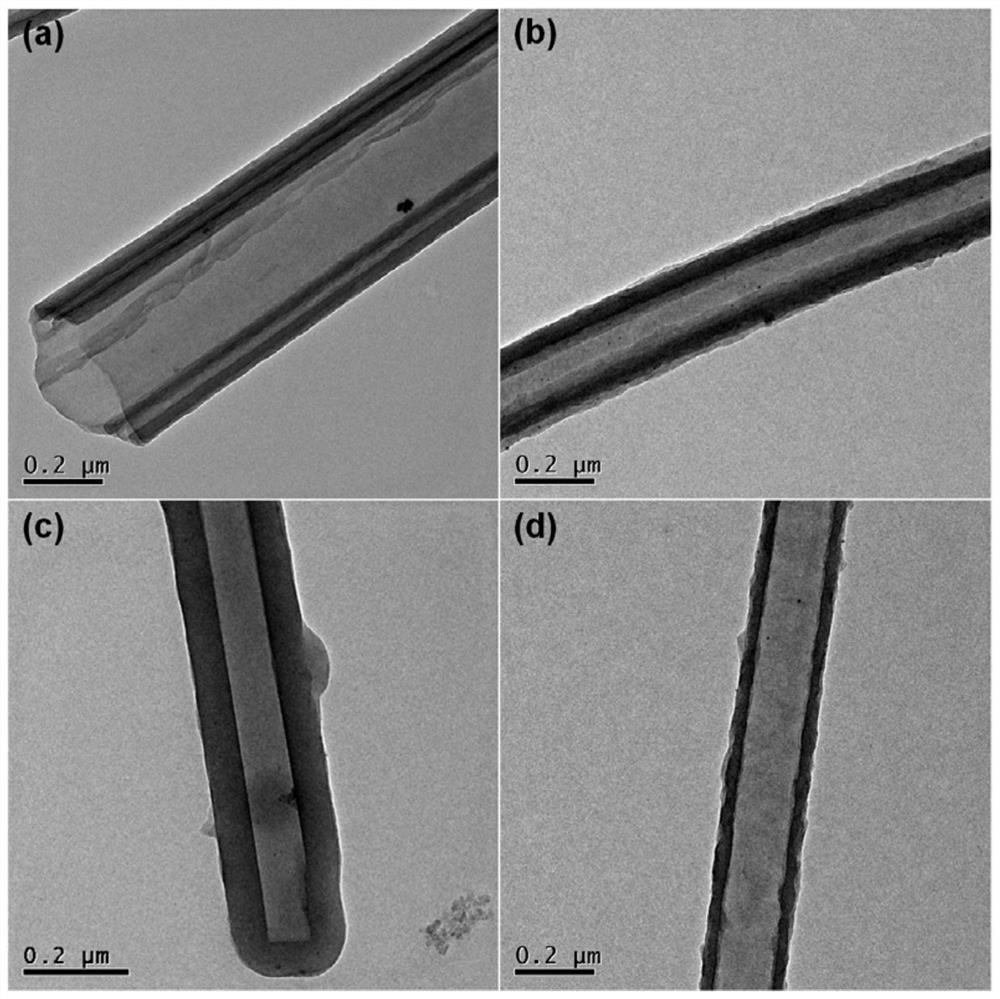
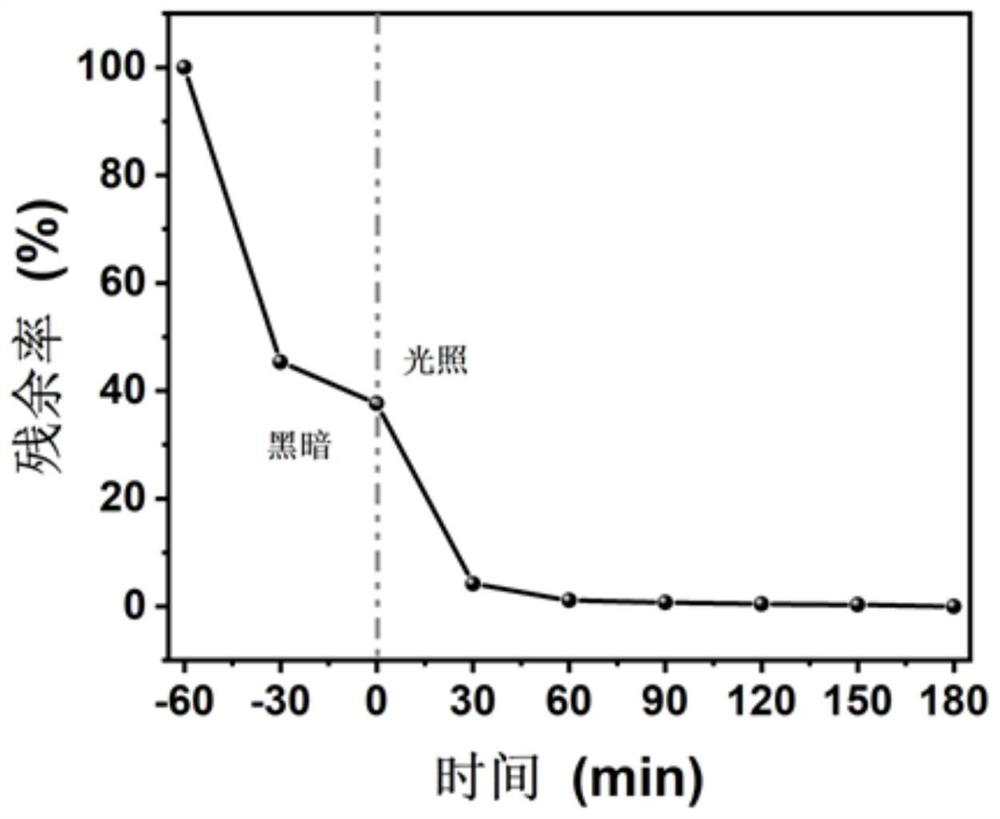
[0055] Add 33.0 mg 5,10,15,20-tetrakis(4-aminophenyl)-porphyrin, 34.5 mg 4,7-bis(4-formylphenyl) to a 10 mL ground-mouth Shrek reaction tube -2,1,3-Benzothiadiazole, o-dichlorobenzene / n-butanol (volume ratio 1 / 1, total amount 4 mL), 0.4 mL 6 mol / L acetic acid aqueous solution as catalyst, after ultrasonic dispersion for 15 min After degassing for 30 min, the reaction tube was depressurized to 50 mtorr by an oil pump under a liquid nitrogen bath, and reacted at 120 °C for 72 h after returning to normal temperature. Naturally cool to room temperature after the end, collect the precipitate by filtration and wash it three times with acetone, then extract it with dioxane and acetone for 24 h to remove unreacted monomers, and obtain the final product, namely the porphyrin-based The conjugated polymer was dried in a vacuum oven at 100 °C for 12 h. Its infrared spectrum is as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com