Application of radiolabeled anti-nano antibody in prognosis and diagnosis of cancer
A nanobody and radionuclide technology, applied in the field of biomedicine or biological imaging, can solve the problem of lack of nanobody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
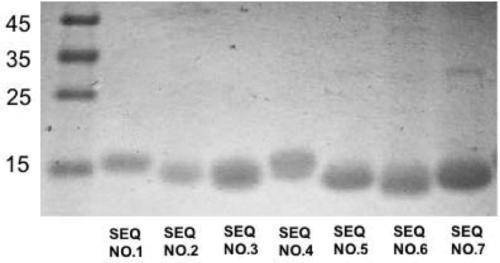
[0151] Example 1 Nanobody expression and purification in host bacteria Escherichia coli
[0152] (1) Electrotransform the corresponding plasmid of the nanobody (sequence shown in SEQ ID NO.: 1-7) into Escherichia coli WK6, and spread it on a culture plate containing ampicillin and glucose in LA+glucose , and cultured overnight at 37°C.
[0153](2) Pick a single colony and inoculate it in 5 mL of LB culture solution containing ampicillin, and cultivate overnight at 37° C. on a shaker.
[0154] (3) Inoculate 1 mL of the overnight strain into 330 mL of TB culture medium, and culture on a shaking table at 37°C. When the OD value reaches 0.6-1, add IPTG, and culture on a shaking table at 28°C overnight.
[0155] (4) Bacteria were collected by centrifugation, and crude antibody extract was obtained by osmosis method.
[0156] (5) Using the infiltration method to obtain the antibody crude extract.
[0157] (6) The nanobody with a purity of more than 90% is prepared by nickel colum...
Embodiment 2
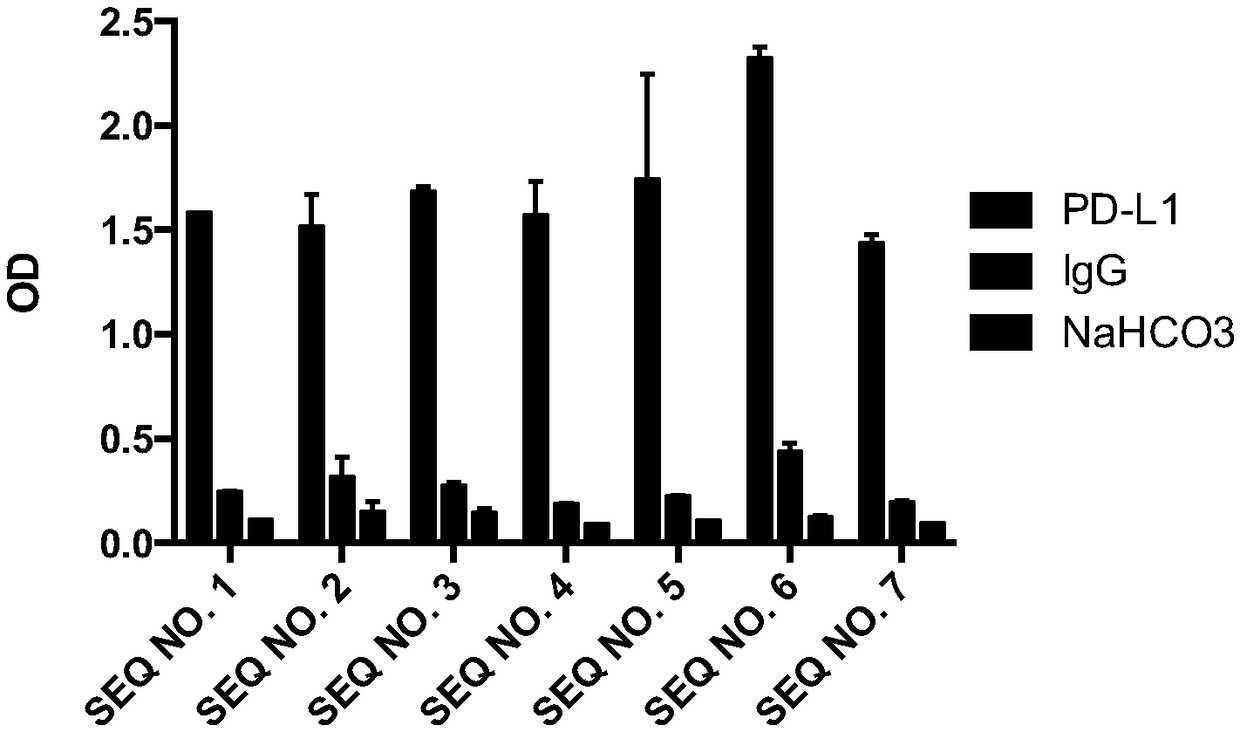
[0159] Embodiment 2 Enzyme-linked immunoassay (ELISA) identifies the affinity of Nanobody
[0160] (1) Antigen protein PD-L1 and IgG coated: 0.5 μg (5 μg / mL, 100 μL) per well, coated with NaHCO 3 (100mM, Ph8.2) was used as blank control, overnight at 4°C.
[0161] (2) The next day, wash with PBST 3 times, add 200 μL of 1% BSA to block for 2 hours at room temperature.
[0162] (3) Dilute each purified nanobody to 10 μg / mL, take 100 μL and incubate with coated PD-L1, PD-L2 and blank control group, and react at room temperature for 1 hour.
[0163] (4) Wash off unbound antibody with PBST, add 100 μL mouse anti-HA tag antibody (diluted 1:2000), and let stand at room temperature for 1 hour.
[0164] (5) Wash off unbound antibody with PBST, add anti-mouse alkaline phosphataseconjugate (diluted 1:2000), and let stand at room temperature for 1 hour.
[0165] (6) Wash off the unbound antibody with PBST, add alkaline phosphatase chromogenic solution, and read the absorbance at a wave...
Embodiment 3
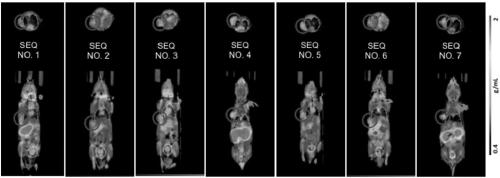
[0171] Example 3 Flow cytometry to detect whether the nanobody has the effect of blocking the binding of PD-1 and PD-L1
[0172] (1) Take 1×10 6 A HEK293F cell transiently expressing the full-length human PD-L1 protein was resuspended in 0.5% BSA-PBS buffer, 10 μg of anti-PD-L1 nanobody was added, and positive control, negative control and blank group (PBS) were set at the same time, and all samples were added 5μg hPD-1-Fc-Biotin, incubated at 4°C for 20min.
[0173] (2) Wash cells twice with PBS, add eBioscience SA-PE, incubate at 4°C for 20 min, wash cells twice with PBS, and detect with flow cytometer.
[0174] The results are shown in Table 1, and the results show that the Nanobodies of the present invention cannot block the binding effect of PD-1 and PD-L1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com