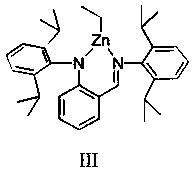
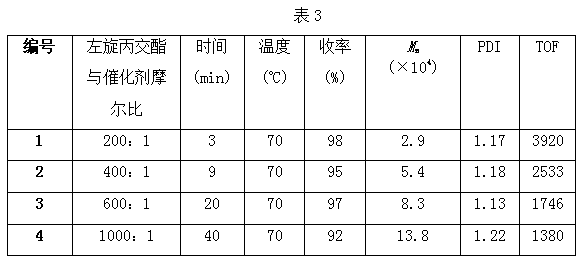
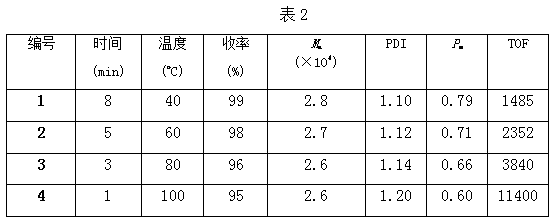
Method of catalyzing polymerization of lactide by using anilido-imine magnesium complex
A technology of amine imine magnesium and complexes, which is applied in the field of catalytic lactide polymerization, can solve the problems of metal residues, and achieve the effects of low cost, controllable molecular weight and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The structural formula of the ligand used is the above formula (A), where R is hydrogen, and the reaction process is as follows: under a nitrogen atmosphere, slowly add 5 mL of benzyl alcohol tetrahydrofuran solution (2.0 mol / L) to an equimolar amount of Mg( n Bu) 2 Hexane solution (2.0 mol / L, 5 mL) was reacted for 1 hour, then 2.72 g of the ligand was dissolved in 15 mL of dry toluene, and added to Mg( n Bu) 2In the reaction mixture with benzyl alcohol, after adding, let the reaction liquid rise to room temperature naturally, then heat to 60°C for 1 hour, after the reaction is completed, the solvent is vacuum-dried, and the residue is washed with dry n-hexane, filtered, and then the product is collected and dried Weighed to obtain 3.43 g of solid, the yield was 85.3%.
Embodiment 2
[0039] The structural formula of the ligand used is the above formula (A), where R is a methyl group, and the reaction process is as follows: under a nitrogen atmosphere, slowly add 5 mL of benzyl alcohol tetrahydrofuran solution (2.0 mol / L) to an equimolar amount of Mg( n Bu) 2 Hexane solution (2.0 mol / L, 5 mL) was reacted for 1 hour, then 3.28 g of the ligand was dissolved in 20 mL of dry toluene, and added to Mg( n Bu) 2 and benzyl alcohol reaction mixture, after adding, the reaction liquid was naturally raised to room temperature, and then heated to 40 ° C for 12 hours, after the reaction was completed, the solvent was vacuum-dried, and the residue was washed with dry n-hexane, filtered, and then the product was collected and dried Weighed to obtain 4.07g of solid, the yield was 88.9%.
Embodiment 3
[0041] The structural formula of the ligand used is the above formula (A), where R is ethyl, and the reaction process is as follows: under a nitrogen atmosphere, slowly add 5 mL of benzyl alcohol tetrahydrofuran solution (2.0 mol / L) to an equimolar amount of Mg( n Bu) 2 Hexane solution (2.0 mol / L, 5 mL) was reacted for 1 hour, then 3.84 g of the ligand was dissolved in 25 mL of dry toluene, and added to Mg( n Bu) 2 and benzyl alcohol reaction mixture, after adding, the reaction solution was naturally raised to room temperature, and then heated to 50 ° C for 3 hours, after the reaction was completed, the solvent was vacuum-dried, and the residue was washed with dry n-hexane, filtered, and then the product was collected and dried Weighed to obtain 4.17g of solid, the yield was 81.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com