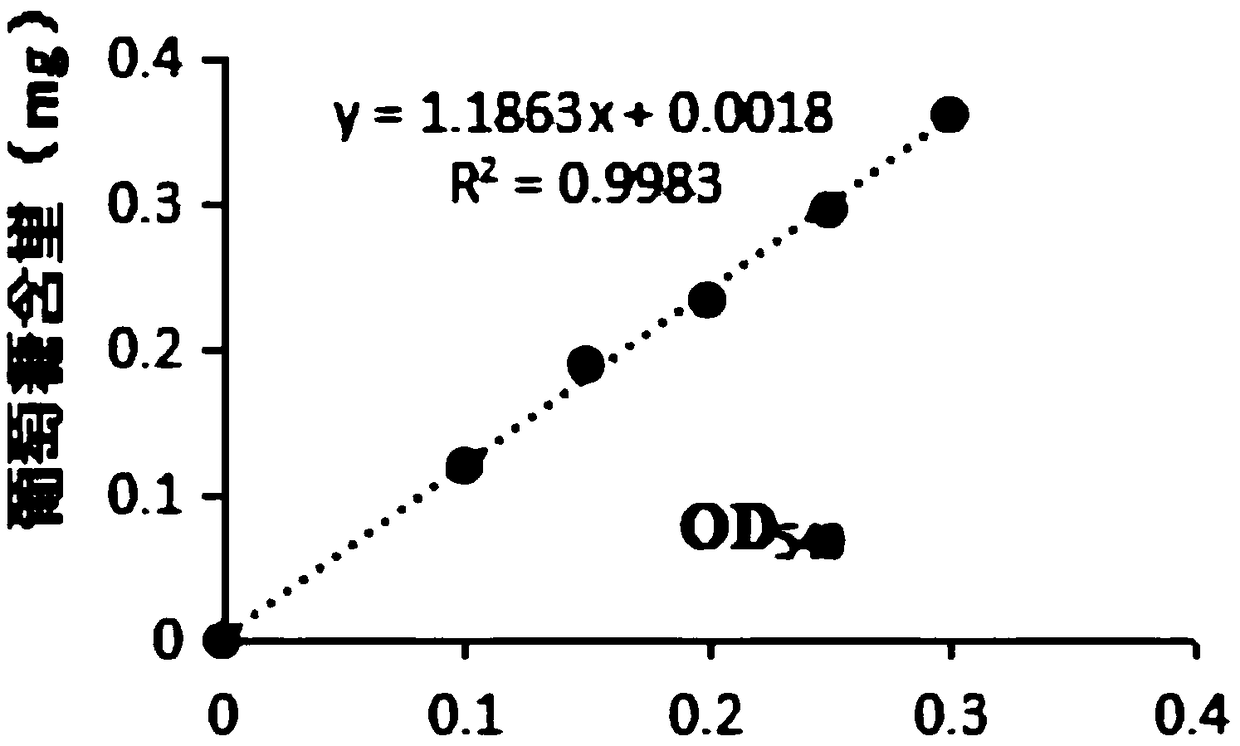
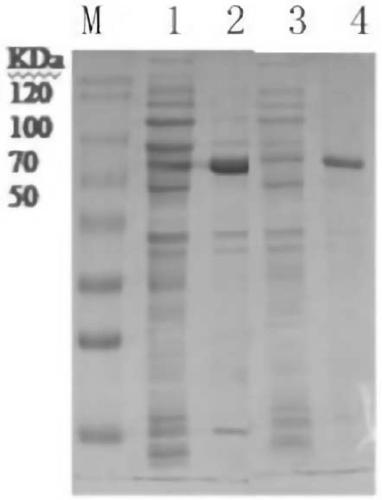
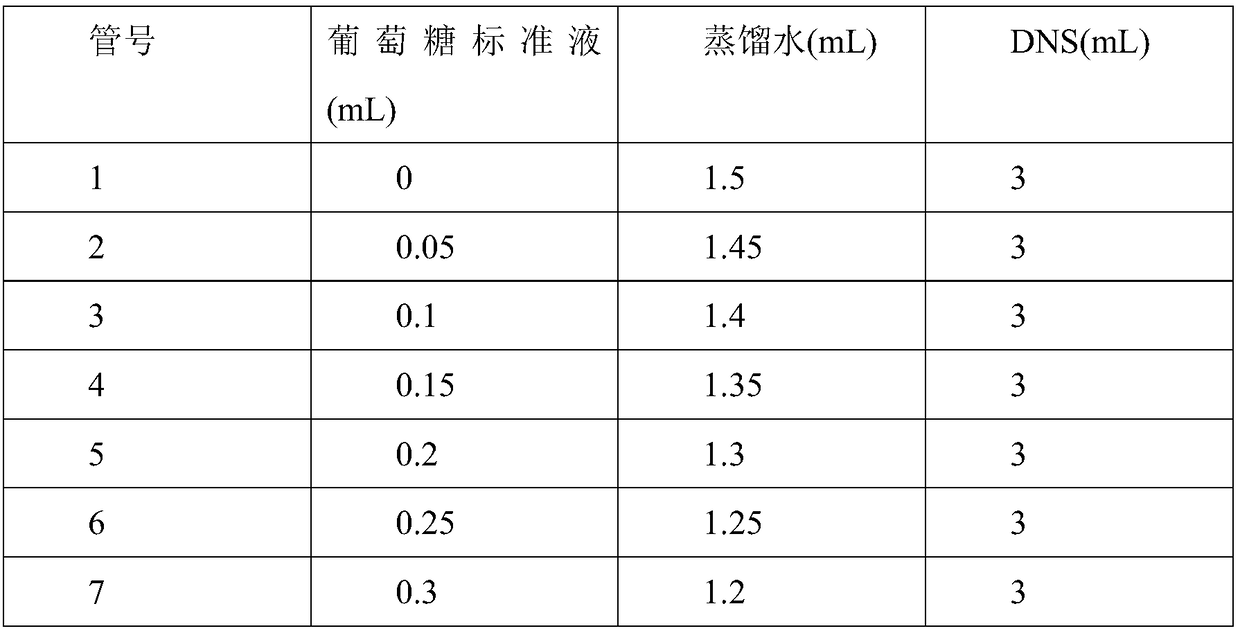
Method for improving sucrose phosphorylase expression efficiency by molecular chaperone co-expression
A technology of sucrose phosphorylase and efficiency, applied in the field of molecular chaperone co-expression to improve the expression efficiency of sucrose phosphorylase, to reduce the formation of inclusion bodies, improve enzyme activity, and promote the effect of increasing the soluble expression level and activity of SPase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1 Construction method of molecular chaperone co-expression system
[0051] 1. Cloning of the sucrose phosphorylase gene
[0052] The sucrose phosphorylase gene was synthesized by a chemical total synthesis method, and its nucleotide sequence is shown in SEQ ID NO.2.
[0053] 2. Construction of recombinant plasmid pET-20b-SPase
[0054] First, pET-20b was double-digested with NcoI and XhoI, and then the synthetic gene fragment was inserted between the two restriction sites of NcoI and XhoI of pET-20b.
[0055] 3. CaCl 2 Preparation of E. coli Competent Cells and Thermal Transformation Steps
[0056] Streak the preserved E.coli BL21 (DE3) glycerol strain on an LB solid plate without an anti-plate, and place the plate upside down in a 37°C incubator for about 12 hours to isolate a single colony. Pick a single colony with neat edges and a moderate size in fresh medium, and culture it in a shaker at 37°C and 180rpm until OD 600 Stop the culture when it is 0.3, p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com