Preparation technique of 1,3-dihydroxyacetone
A technology for the preparation of dihydroxyacetone, which is applied in the field of preparation of 1,3-dihydroxyacetone, can solve the problems of Au sintering and particle enlargement, insufficient activity, and low utilization rate of active components, etc., to increase the surface area and Stability, enhanced activity and selectivity, and the effect of enriching active sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
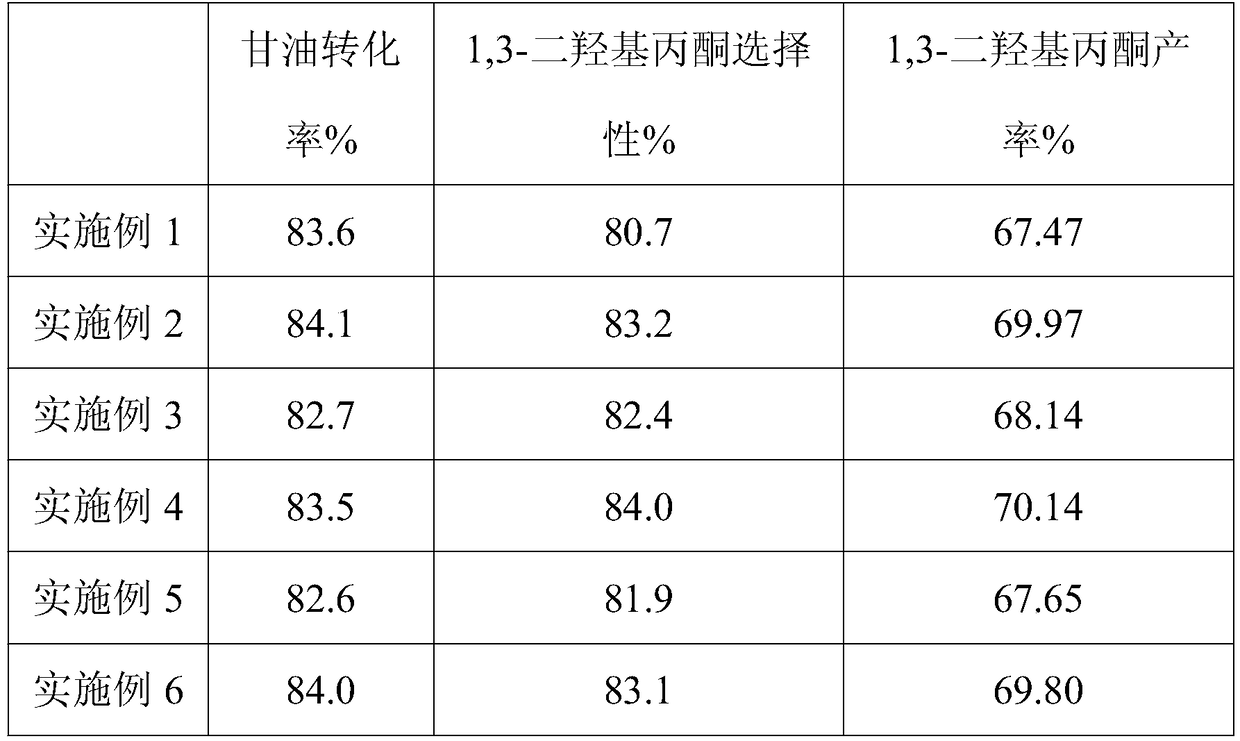
[0023] A preparation process of 1,3-dihydroxyacetone, the specific steps are as follows: add glycerin aqueous solution and catalyst into the high-pressure reaction kettle, after the airtightness is complete, use high-purity oxygen to evacuate three times at room temperature, and then fill it with 1.0MPa high-pressure reactor. Pure oxygen, stirred and heated to 80°C, reacted at constant temperature for 3 hours, cooled to room temperature with ice bath, centrifuged and filtered, and the reaction liquid was detected by high performance liquid chromatography to measure the conversion rate of glycerol and the selectivity of 1,3-dihydroxyacetone;
[0024] The preparation method of catalyst described in the present embodiment is:
[0025] (1) Preparation of ZnAl-HTLc: soluble 0.375gAl(NO 3 ) 3 9H 2 O, 0.891gZn(NO 3 ) 2 ·6H 2 O was dissolved in 150ml of deionized water to prepare mixed solution A, and 0.042gNa 2 CO 3 Dissolve 0.48g NaOH in 150ml deionized water to prepare mixed...
Embodiment 2
[0029] A preparation process of 1,3-dihydroxyacetone, the specific steps are as follows: add glycerin aqueous solution and catalyst into the high-pressure reaction kettle, after the airtightness is complete, use high-purity oxygen to evacuate three times at room temperature, and then fill it with 1.0MPa high-pressure reactor. Pure oxygen, stirred and heated to 80°C, reacted at constant temperature for 3 hours, cooled to room temperature with ice bath, centrifuged and filtered, and the reaction liquid was detected by high performance liquid chromatography to measure the conversion rate of glycerol and the selectivity of 1,3-dihydroxyacetone;
[0030] The preparation method of catalyst described in the present embodiment is:
[0031] (1) Preparation of ZnAl-HTLc: soluble 0.375gAl(NO 3 ) 3 9H 2 O, 0.594gZn(NO 3 ) 2 ·6H 2 O was dissolved in 150ml of deionized water to prepare mixed solution A, and 0.042gNa 2 CO 3 Dissolve 0.32g NaOH in 150ml deionized water to prepare mixed s...
Embodiment 3
[0035] A preparation process of 1,3-dihydroxyacetone, the specific steps are as follows: add glycerin aqueous solution and catalyst into the high-pressure reaction kettle, after the airtightness is complete, use high-purity oxygen to evacuate three times at room temperature, and then fill it with 1.0MPa high-pressure reactor. Pure oxygen, stirred and heated to 80°C, reacted at constant temperature for 3 hours, cooled to room temperature with ice bath, centrifuged and filtered, and the reaction liquid was detected by high performance liquid chromatography to measure the conversion rate of glycerol and the selectivity of 1,3-dihydroxyacetone;
[0036] The preparation method of catalyst described in the present embodiment is:
[0037] (1) Preparation of ZnAl-HTLc: soluble 0.375gAl(NO 3 ) 3 9H 2 O, 0.891gZn(NO 3 ) 2 ·6H 2 O was dissolved in 150ml of deionized water to prepare mixed solution A, and 0.05gNa 2 CO 3 Dissolve 0.40g NaOH in 150ml deionized water to prepare mixed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com