Microchannel reaction method of 4-chloroethyl acetoacetate and device
A technology of ethyl chloroacetoacetate and microchannel reaction, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and preparation of ketene/polyketene, etc., which can solve the problems of difficult disposal of solid waste, increased production costs, and by-products increase and other problems, to achieve the effect of easy control of reaction temperature, high production capacity and sufficient reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
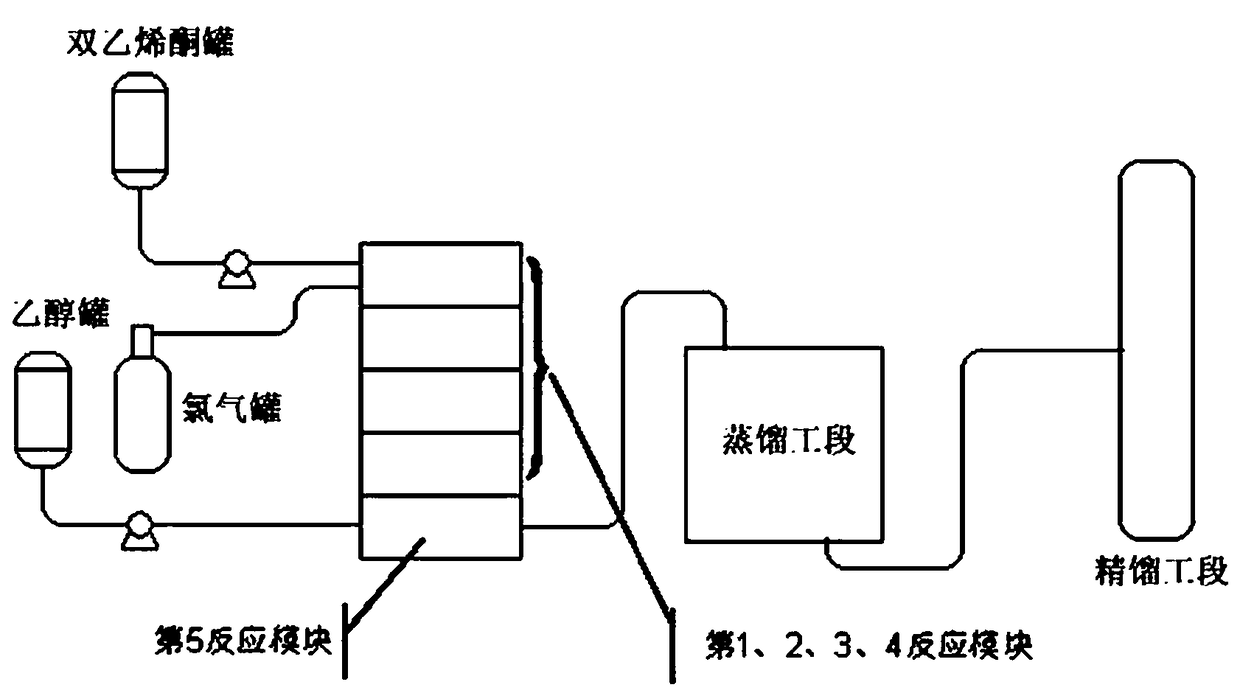
[0018] A kind of microchannel reaction method of ethyl 4-chloroacetoacetate, concrete implementation steps are as follows:
[0019] 1. Open the refrigerant valves of the 1st, 2nd, 3rd, and 4th reaction modules of the microchannel reactor, close the refrigerant valve of the 5th reaction module, control the refrigeration temperature at 3 ° C, diketene and dichloromethane according to the volume ratio of 2:1 Mix in the diketene tank first, then enter the first reaction module of the microchannel reactor at a flow rate of 10mL / min after mixing, and simultaneously pass chlorine gas with a pressure of 0.25Mpa and a flow rate of 1500mL / min into the first reaction module of the microchannel reactor. Chlorination reaction;
[0020] 2. When the chlorination reaction solution from the fourth reaction module reaches the fifth reaction module, add absolute ethanol to the fifth reaction module, control the flow rate at 6mL / min, and control the reaction temperature of the fifth reaction modu...
example 2
[0023] 1. The refrigerant valve of the 1st, 2, 3, 4 reaction modules of the microchannel reactor is opened, the refrigerant valve of the 5th reaction module is closed, the refrigeration temperature is controlled at -8 ℃, diketene and dichloromethane are according to volume ratio 3: 1 Mix in a diketene tank first, then enter the first reaction module of the microchannel reactor at a flow rate of 15mL / min after mixing, and simultaneously pass chlorine gas with a pressure of 0.28Mpa and a flow rate of 3000mL / min into the first reaction module of the microchannel reactor carry out chlorination reaction;
[0024] 2. When the chlorination reaction solution from the fourth reaction module reaches the fifth reaction module, add absolute ethanol to the fifth reaction module, control the flow rate at 8mL / min, and control the reaction temperature of the fifth reaction module at 60°C;
[0025] 3. The esterification reaction solution from the fifth reaction module is firstly distilled (at ...
example 3
[0027] 1. Open the refrigerant valves of the 1st, 2nd, 3rd and 4th reaction modules of the microchannel reactor, close the refrigerant valves of the 5th reaction module, and control the refrigeration temperature at -15°C. Diketene and methylene chloride are in a volume ratio of 3.5: 1 Mix in the diketene tank first, then enter the first reaction module of the microchannel reactor at a flow rate of 20mL / min after mixing, and simultaneously pass chlorine gas with a pressure of 0.30Mpa and a flow rate of 4000mL / min into the first reaction module of the microchannel reactor carry out chlorination reaction;
[0028] 2. When the chlorination reaction solution from the fourth reaction module reaches the fifth reaction module, add absolute ethanol to the fifth reaction module, control the flow rate at 10mL / min, and control the reaction temperature of the fifth reaction module at 50°C;
[0029] 3. The esterification reaction solution from the fifth reaction module is firstly distilled ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com