Preparation method of pregabalin
A technology of pregabalin and chiral ester, applied in the field of preparation of pregabalin, can solve problems such as low yield and severe reaction conditions, and achieve the effects of improving purity, convenient operation, and safe and environmentally friendly preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
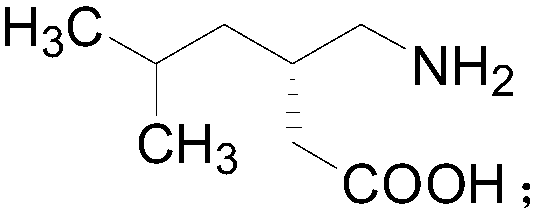
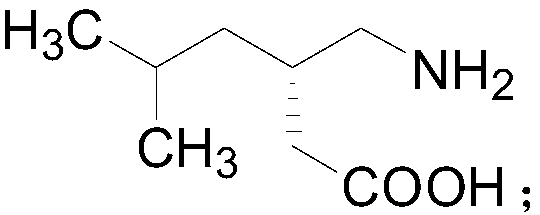
[0020] The present invention provides a kind of preparation method of pregabalin, and the structural formula of pregabalin is as follows:
[0021]
[0022] Wherein, the preparation method of the pregabalin shown in the above structural formula is: using the pregabalin racemate as the starting material, under the action of lipase to prepare the pregabalin chiral ester, and the pregabalin chiral ester is hydrolyzed The reaction gives pregabalin.
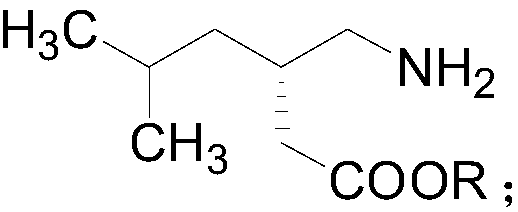
[0023] The structural formula of pregabalin chiral ester is as follows:
[0024]
[0025] Wherein, R is selected from methyl, ethyl or isopropyl.
[0026] The reaction process of the chiral ester of pregabalin is as follows: adding solvent and pregabalin racemate to the reaction bottle, then adding lipase, constant temperature shaker, 200rpm reaction for 24h, suction filtration, the filtrate is concentrated by silica gel column chromatography, and the corresponding eluted solution, concentrated to give pregabalin chiral ester. ...
Embodiment 1
[0029] This embodiment discloses a kind of pregabalin prepared according to the above method, wherein R is selected from ethyl, and the specific reaction steps are as follows:
[0030] Add 100ml of ethanol and 12.5g of pregabalin racemate to a 500ml round bottom flask, then add 1g of lipase, react in a constant temperature shaker at 40°C, 200rpm for 24h, filter with suction, concentrate the filtrate by silica gel column chromatography, and collect the corresponding eluent. After concentration, 6.1 g of chiral ester of pregabalin was obtained.
[0031] Add 5g of pregabalin chiral ester and 10ml of concentrated hydrochloric acid into a 50ml reaction bottle, stir at reflux for 3h, adjust to PH5 with 2M sodium hydroxide, dissolve and dry dichloromethane after concentration, then remove dichloromethane under reduced pressure, and recrystallize with ethanol / acetone , to obtain high-purity pregabalin 3.2g.
[0032] The purity of the high-purity pregabalin finally prepared in this ex...
Embodiment 2
[0034] This embodiment discloses a kind of pregabalin prepared according to the above method, wherein R is selected from isopropyl, and the specific reaction steps are as follows:
[0035] Add 100ml of isopropanol and 12.5g of pregabalin racemate to a 500ml round bottom flask, then add 1g of lipase, and react on a constant temperature shaker at 40°C at 200rpm for 24h. After suction filtration, the filtrate was concentrated by silica gel column chromatography, and the corresponding eluate was collected and concentrated to obtain 6.2 g of pregabalin chiral ester.
[0036] Add 5g of pregabalin chiral ester and 20ml of 2M sodium hydroxide to a 50ml reaction bottle, stir at 80°C for 6h, adjust to PH5 with 6M hydrochloric acid, dissolve ethyl acetate after concentration and dry, then remove dichloromethane under reduced pressure, ethanol / acetone weight Crystallized to obtain 3.3 g of high-purity pregabalin.
[0037] The purity of the high-purity pregabalin finally prepared in this ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com