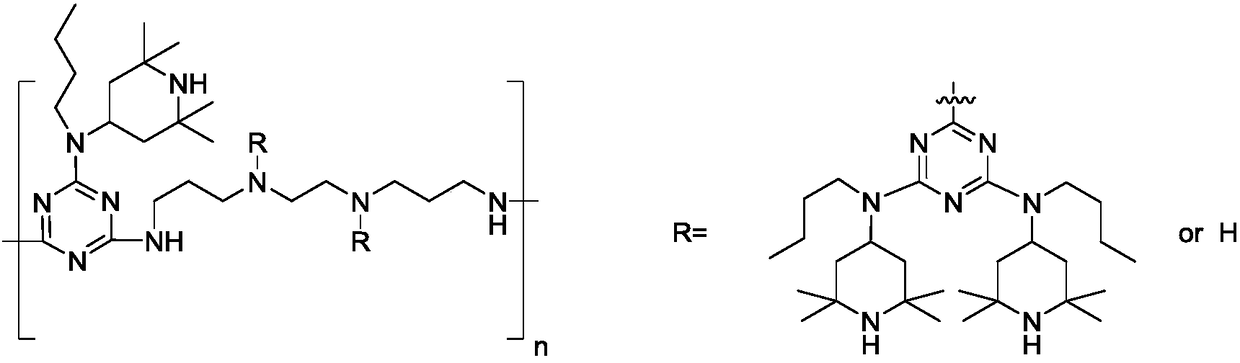
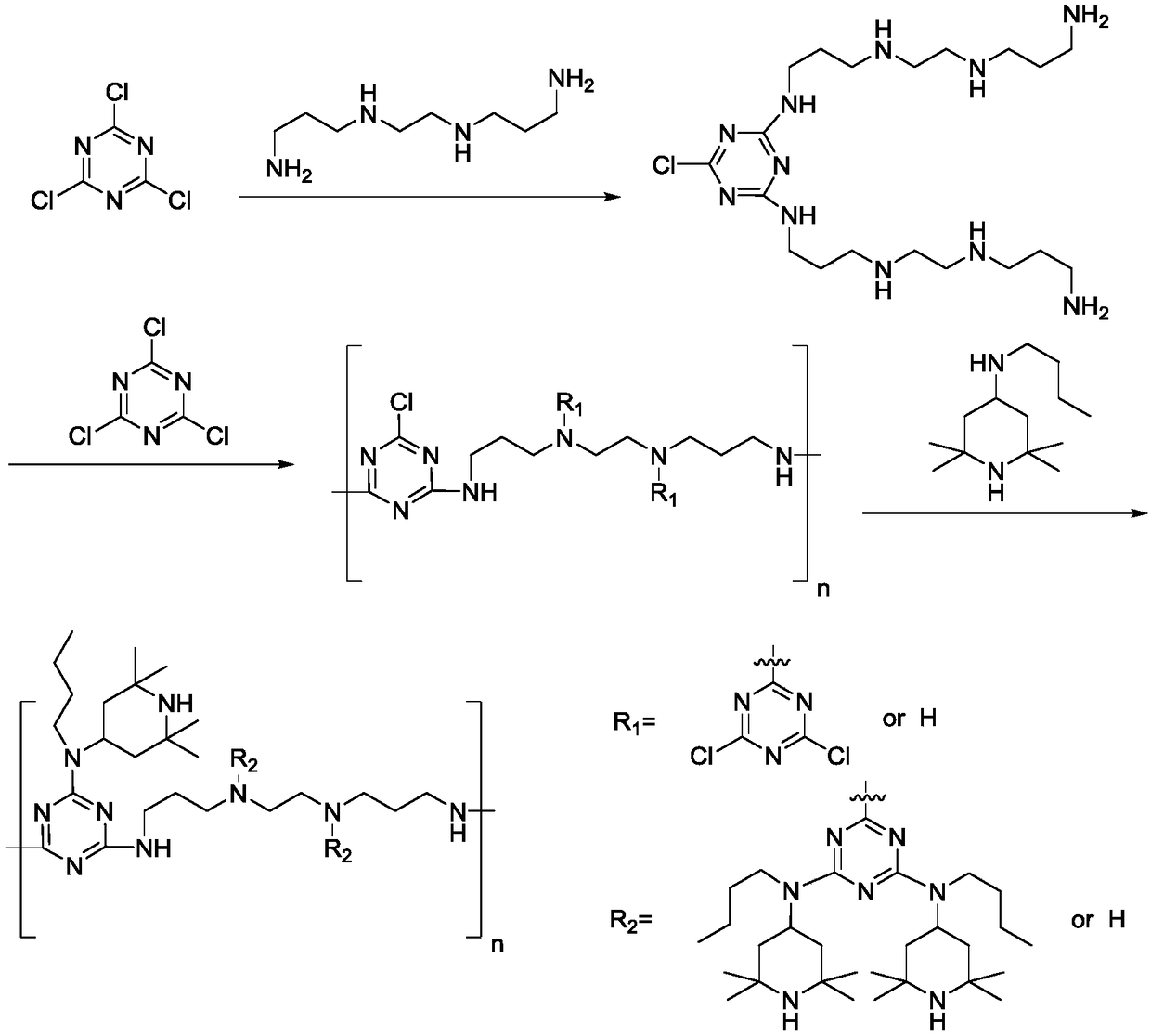
Preparation method of polymeric type HALS (Hindered Amine Light Stabilizer) HA-88
A technology of HA-88 and hindered amines, which is applied in the field of functional additives for polymer materials, can solve problems such as the difficulty of molecular weight regulation, achieve the effect of optimizing molecular weight distribution and improving the difficulty of molecular weight regulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The preparation of embodiment 1 HA-88
[0032]Add 1.39g (8mmol) N,N'-bis(3-aminopropyl)ethylenediamine and 20mL toluene to the three-necked flask, drop 0.74g (4mmol) trimeric Add 0.9 mL of 30% sodium hydroxide aqueous solution to the reaction liquid after the dropwise addition of 5 mL of toluene solution of cyanogen chloride, continue to react in ice bath for 2 h, then raise the temperature to 45° C. for 4 h. After the reaction of this stage was completed, the ice bath was down to 10°C, and 25mL of toluene solution of 3.69g (20mmol) cyanuric chloride was added dropwise thereto. Insulation reaction under the bath for 6h. After the reaction at this stage is completed, add 9.34g (44mool) N-butyl-2,2,6,6-tetramethyl-4-piperidinamine and 4.5mL 30% sodium hydroxide aqueous solution to the system, and heat up to 45 °C for 3 hours, then continue to heat up to reflux for 4 hours. The reaction solution was washed with water and the solvent was distilled off under reduced press...
Embodiment 2
[0034] The preparation of embodiment 2 HA-88
[0035] Add 1.39g (8mmol) N,N'-bis(3-aminopropyl)ethylenediamine and 20mL xylene to the three-necked flask, drop 0.74g (4mmol) Tris Add 0.7 g (8.3 mmol) of sodium bicarbonate to the reaction liquid after the dropwise addition of 5 mL of toluene solution of polycyanogen chloride, continue to react in ice bath for 2 h, then raise the temperature to 45° C. for 4 h. After the reaction of this stage was completed, the ice bath was down to 10°C, and 25mL of toluene solution of 2.95g (16mmol) cyanuric chloride was added dropwise thereto, and 1.4g (16.6mmol) sodium bicarbonate was added in the reaction solution after the dropwise addition. The reaction was incubated for 8 hours in an ice bath. After the reaction at this stage was completed, 7.65g (36mool) N-butyl-2,2,6,6-tetramethyl-4-piperidinamine and 3.1g (37mmol) sodium bicarbonate were added to the system, and the temperature was raised to 45 °C for 3 hours, then continue to heat up...
Embodiment 3
[0037] The preparation of embodiment 3 HA-88
[0038] Add 1.39g (8mmol) N,N'-bis(3-aminopropyl)ethylenediamine and 20mL xylene to the three-necked flask, drop 0.81g (4.4mmol) into the solution after the ice bath is lowered to 10°C After the 5mL toluene solution of cyanuric chloride was added dropwise, 0.9mL of 30% sodium hydroxide aqueous solution was added to the reaction solution, and the reaction was continued for 2h under ice-bath conditions, and then the temperature was raised to 45°C for 4h. After the completion of the reaction at this stage, the ice bath was down to 10°C, and 25mL of toluene solution of 3.32g (18mmol) cyanuric chloride was added dropwise thereto, and 2.2mL of 30% aqueous sodium hydroxide solution was added to the reaction solution after the dropwise addition was completed. Insulation reaction under the bath for 6h. After the reaction at this stage is completed, add 8.58g (40.4mool) N-butyl-2,2,6,6-tetramethyl-4-piperidinamine and 4.5mL30% sodium hydrox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com