Preparation method of nano-palladium catalyst with controllable morphology and size
A nano-palladium and catalyst technology, applied in the field of preparation of nano-palladium catalysts, can solve the problems of short preparation period, mild reaction conditions and the like, and achieve the effects of short preparation period, mild reaction conditions and good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] At room temperature, weigh 3 mg of palladium chloride into a test tube, add 3 mL of dichloromethane, stir until completely dissolved, the solution is bright yellow, and obtain a palladium precursor solution;
[0034] Then add 10 mg of oleic acid as a dispersant to the palladium precursor solution, continue stirring, and then drop 10 μL of phenylsilane into the reaction system, and react for 2 hours to obtain a black colloidal solution;
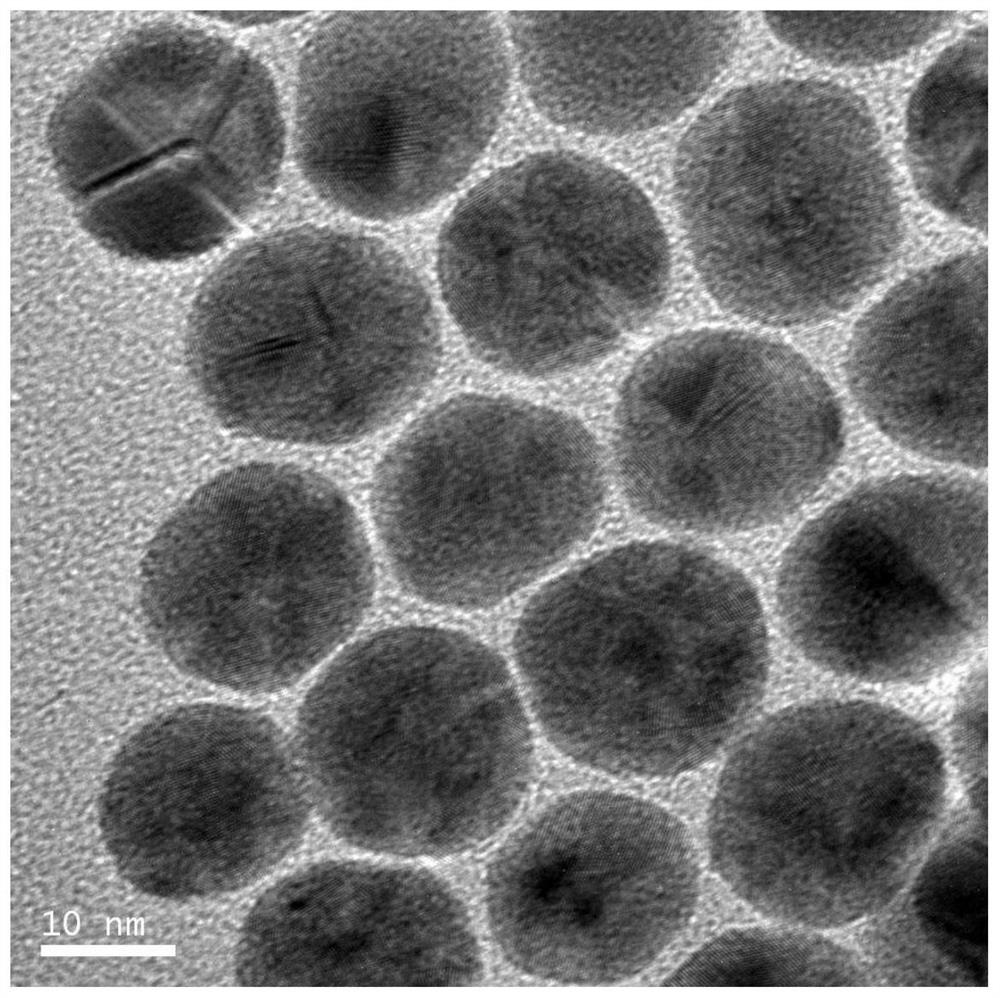
[0035] Add ethanol to the black colloidal solution to induce aggregation, centrifuge to collect the black product, wash 3 times with ethanol-dichloromethane (volume ratio of ethanol and dichloromethane is 1:1) solution, and then redisperse in dichloromethane to obtain The nano-palladium catalyst, the microscopic appearance is as figure 1 As shown, the nano-palladium particle morphology is spherical, and the particle size is 10nm.
Embodiment 2
[0037] At room temperature, weigh 3 mg of palladium chloride into a test tube, add 3 mL of dichloromethane, stir until completely dissolved, the solution is bright yellow, and obtain a palladium precursor solution;
[0038] Then, 15 mg of oleic acid was added into the palladium precursor solution as a dispersant, and the stirring was continued, and 15 μL of diphenylsilane was added dropwise into the reaction system. After 2 hours of reaction, a black colloidal solution was obtained.
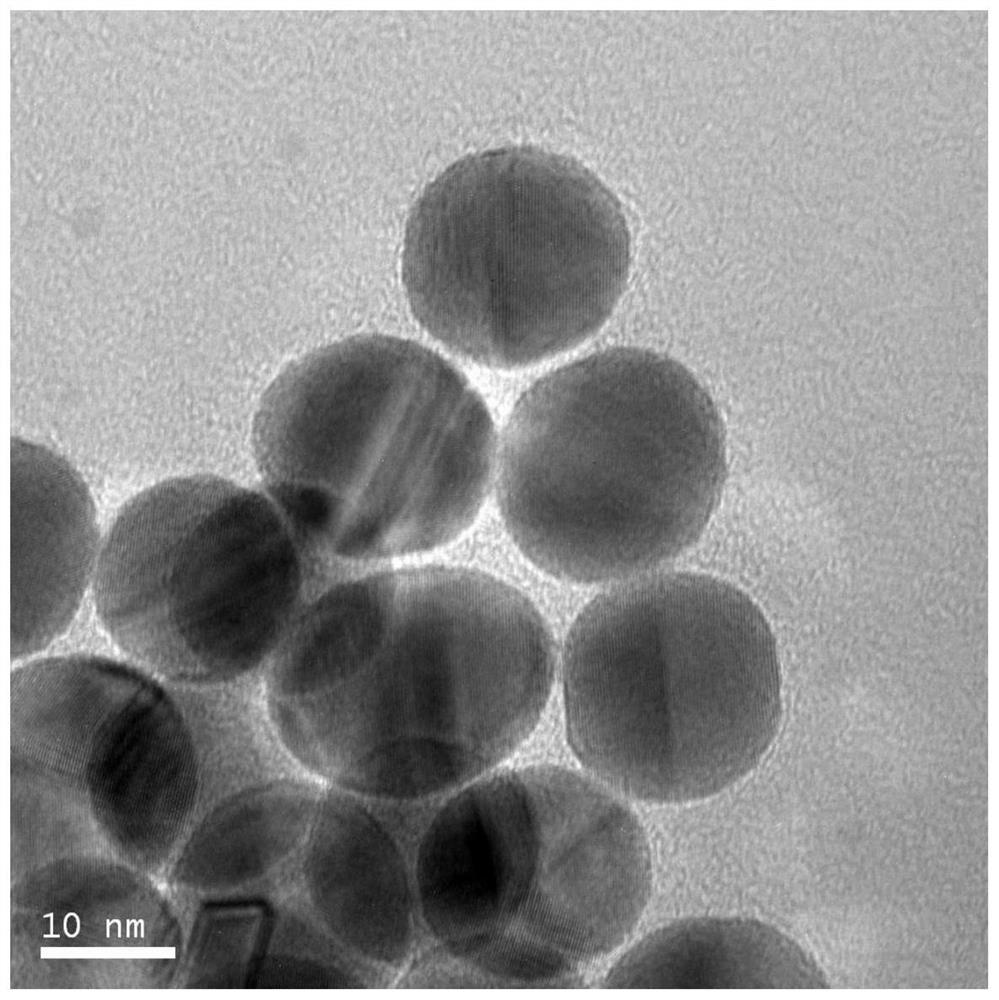
[0039] Add ethanol to the black colloidal solution to induce aggregation, centrifuge to collect the black product, wash 3 times with ethanol-dichloromethane (volume ratio of ethanol and dichloromethane is 1:1) solution, and then redisperse in dichloromethane to obtain The nano-palladium catalyst, the microscopic appearance is as figure 2 As shown, the morphology of nano-palladium particles is ellipsoidal, and the particle size is 15nm.
Embodiment 3
[0041] At room temperature, weigh 3 mg of sodium chloropalladate in a test tube, add 3 mL of dichloromethane, stir until completely dissolved, the solution is bright yellow, and obtain a palladium precursor solution;
[0042] Then, 25 mg of oleic acid was added into the palladium precursor solution as a dispersant, and the stirring was continued, and 30 μL of triphenylsilane was added dropwise into the reaction system. After 2 hours of reaction, a black colloidal solution was obtained.
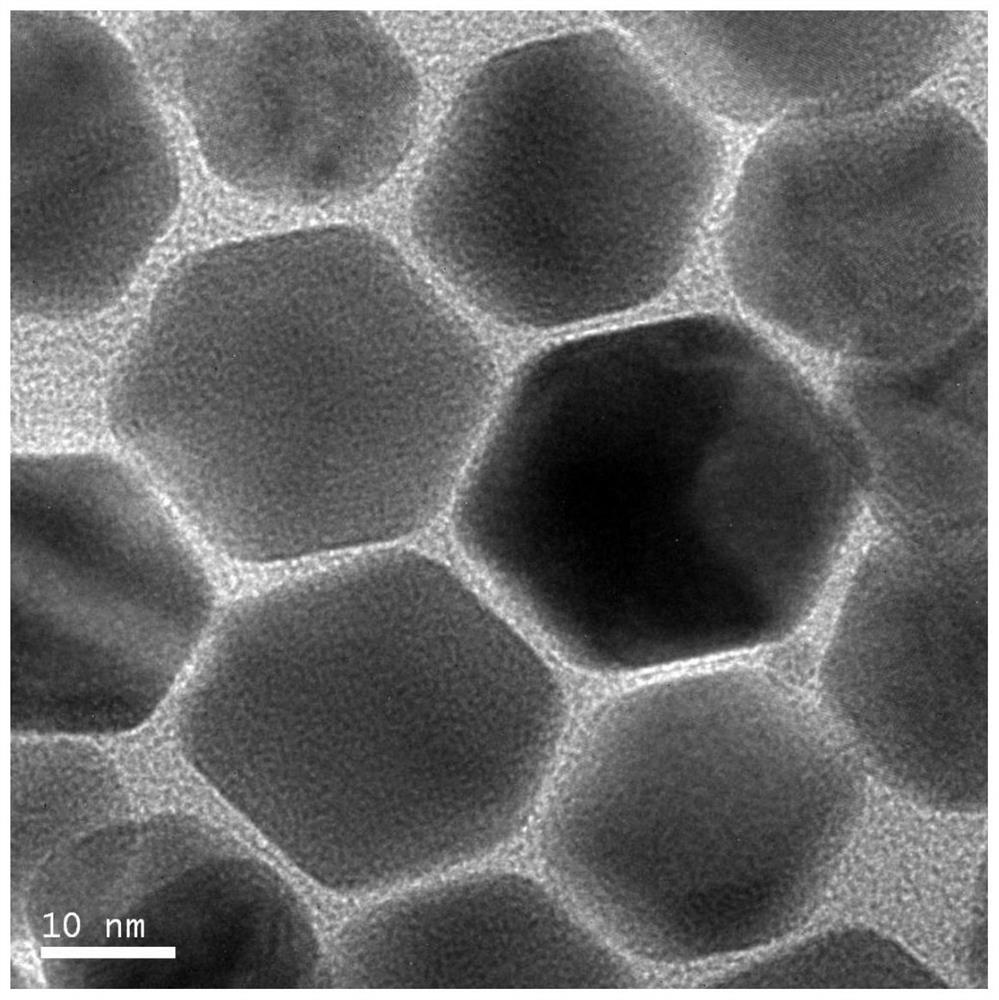
[0043] Add ethanol to the black colloidal solution to induce aggregation, centrifuge to collect the black product, wash 3 times with ethanol-dichloromethane (volume ratio of ethanol and dichloromethane is 1:1) solution, and then redisperse in dichloromethane to obtain The nano-palladium catalyst, the microscopic appearance is as image 3 As shown, the morphology of nano-palladium particles is hexagonal, and the particle size is 15nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com