SIRP alpha mutant or fusion protein thereof, and application thereof
A fusion protein and variant technology, applied in the fields of tumor therapy and molecular immunology, can solve problems such as off-target toxicity, effectiveness and production cost limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1 Construction of expression vector of SIRPα variant-Fc fusion protein
[0095] The amino acid sequences of the wild-type SIRPα and its variants prepared in the present invention are respectively shown in SEQ ID NO.1-3, wherein the amino acid sequence of the wild-type SIRPα, that is, the SIRPα parent, is SEQ ID NO.1; the amino acid sequence of the variant B2F6 It is SEQ ID NO.2; the amino acid sequence of the variant D1H4 is SEQ ID NO.3.
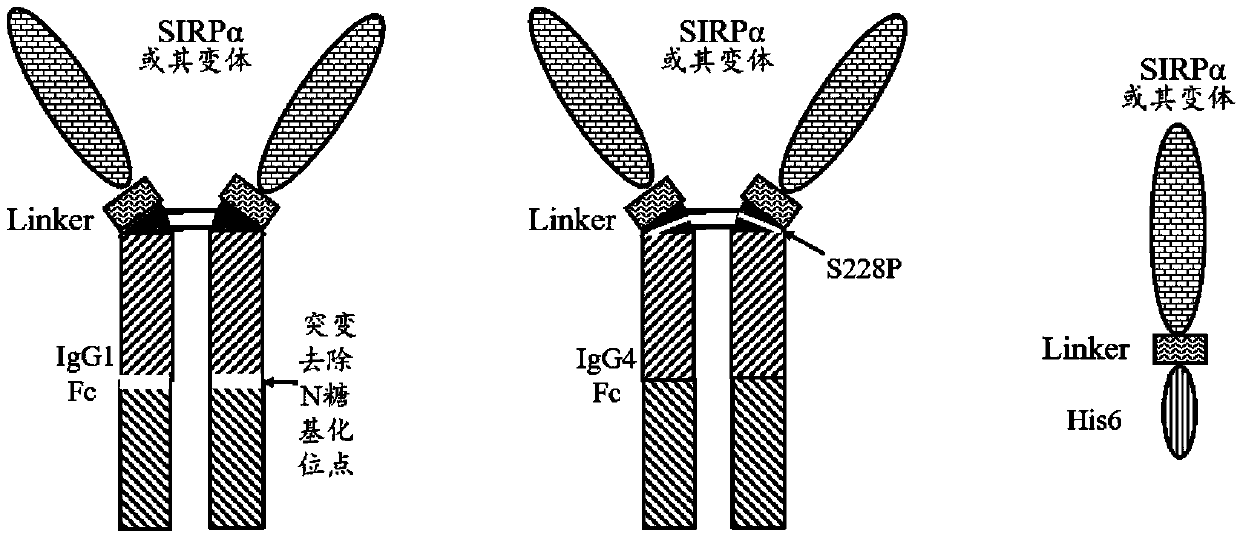
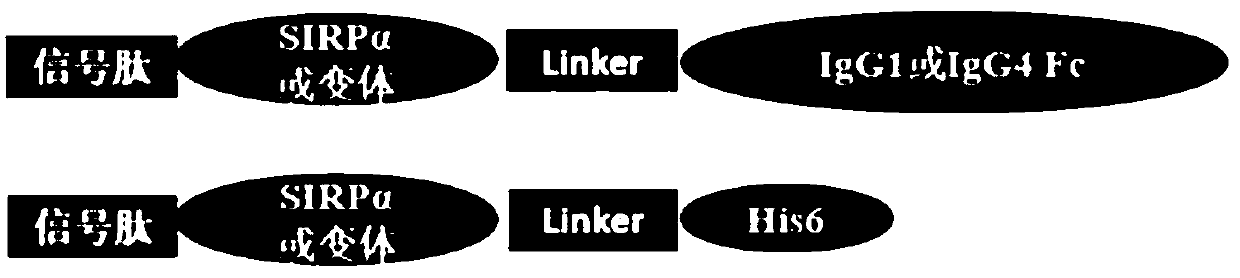
[0096] SIRPα and its variants are connected to IgG1Fc (amino acid sequence: SEQ ID NO.6), IgG4Fc (amino acid sequence: SEQ ID NO.7) or 6×His ( Amino acid sequence: SEQ ID NO.8) etc. are connected to form a fusion protein, and the schematic diagram of its protein structure is as follows figure 1 shown.
[0097] In order to secrete and express SIRPα and its variants in mammalian cells, it is necessary to add a secreted and expressed signal peptide (amino acid sequence: SEQ ID NO.5) to the N segment of the expression vector whe...
Embodiment 2
[0101] Example 2 Expression and purification of SIRPα variant-Fc fusion protein
[0102] Recombinant immunoglobulin variants were expressed by transient transfection of 293E cells.
[0103] 293E cells in the logarithmic growth phase were treated with 4×10 5 / ml density inoculated in a shaker flask at 37°C, 5% CO 2 Shaking, 125r / min cultured for 24 hours before transfection.
[0104] For every 100ml of 293E cells, take 5mL of OPTI-MEM and add 200μg of plasmid, shake and mix and incubate at room temperature for 5min to get the plasmid solution; take another 5mL of OPTI-MEM and add 600μg of PEI, shake and mix and incubate at room temperature for 5min to get the PEI solution ; Mix the plasmid and PEI solution, vortex and incubate at room temperature for 20 minutes, add the reaction mixture dropwise to the cells, and place at 37°C, 5% CO 2 The shaker, cultured at 125r / min, feed fed on the 4th and 6th day, harvested the supernatant on the 8th day.
[0105] Cell culture supernatant...
Embodiment 3
[0106] Example 3 Analysis of binding activity between SIRPα variant-Fc fusion protein and CD47
[0107] Dilute the CD47 antigen to 1 μg / mL in the coating solution, add 100 μL per well to the enzyme-linked plate, and place it in a humid box at 4 °C overnight. Wash the enzyme-linked plate 3 times with a plate washer, block with 1.5% casein, 200 μL per well, and block for 1 hour at 37°C in a humid box. Dilute the fusion protein of SIRPα and its variants with 1×PBS to 5 μg / mL, and after 4-fold gradient dilution, add 100 μL per well to the enzyme-linked plate, and react in a wet box at 37°C for 1 h. Wash the enzyme-linked plate 3 times, add goat anti-human Fc-HRP secondary antibody and react at room temperature for 45 minutes. Wash the enzyme-linked plate 5 times and add 100 μL TMB substrate for color development, react for 3 minutes and use 100 μL 2N H 2 SO 4 The reaction was terminated, and the enzyme-linked immunosorbent assay was read at 450nm.
[0108] The antibody-antigen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com