Novel temperature-resisting nanometer medicine-carried system
A nano-drug loading and temperature-resistant technology, which is applied in the direction of microcapsules, anti-tumor drugs, drug combinations, etc., can solve the problems of not being able to obtain a large amount at one time, heat intolerance, and high cost, and achieve a mature extraction method and low cost , improve the release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Preparation of lipid microbubbles in milk: the milk is divided into centrifuge tubes, and the above lipid microbubbles are collected by centrifugation, including: first centrifuging at 4 degrees for 30 minutes with a centrifugal force of 13000g, Remove fat globules, casein and debris, centrifuge the obtained supernatant at 20,000 g for 1 hour, and collect the obtained precipitate as lipid microvesicles.
Embodiment 2
[0030] Example 2: Preparation of lipid microbubbles in milk: filter the milk through a polypropylene filter membrane with a pore size of 10 microns. The filtered supernatant was centrifuged at a centrifugal force of 20,000 g for 1 hour, and the obtained precipitate was collected as lipid microvesicles. The centrifugation conditions can be between 0.5-2 hours, and the obtained lipid microvesicles are basically similar.
[0031] The lipid microbubble effect that embodiment 1-2 prepares is similar, and effect explanation:
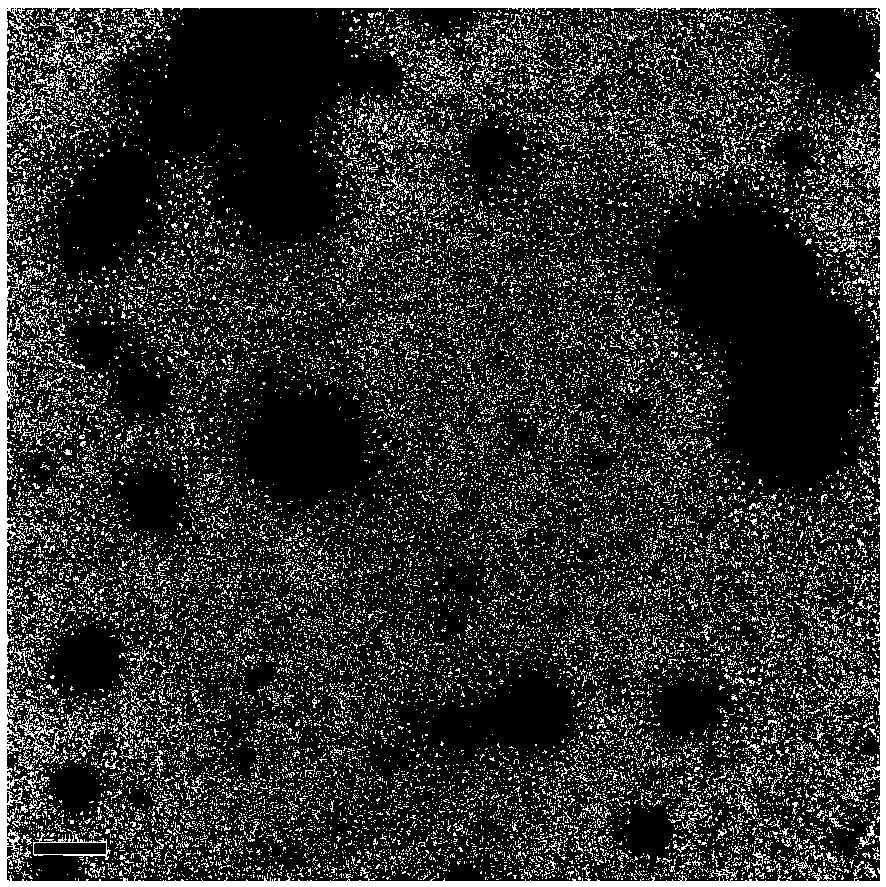
[0032] 1. Observing under a transmission electron microscope after the lipid microbubbles collected are resuspended with 0.9% (g / ml) physiological saline, from figure 1 It can be seen that the particle size range of lipid microbubbles is about 150 nanometers, and the uniformity is high, forming a bubble shape.
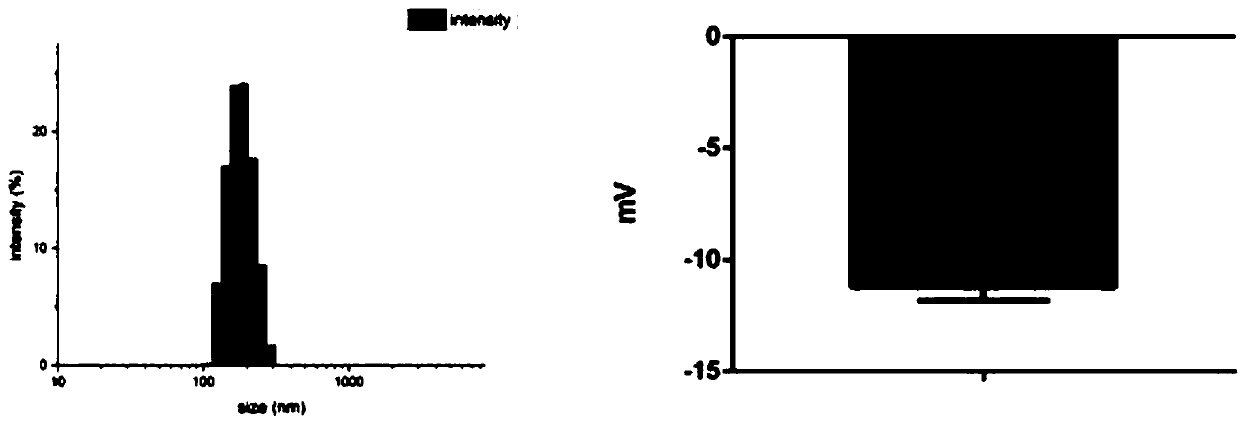
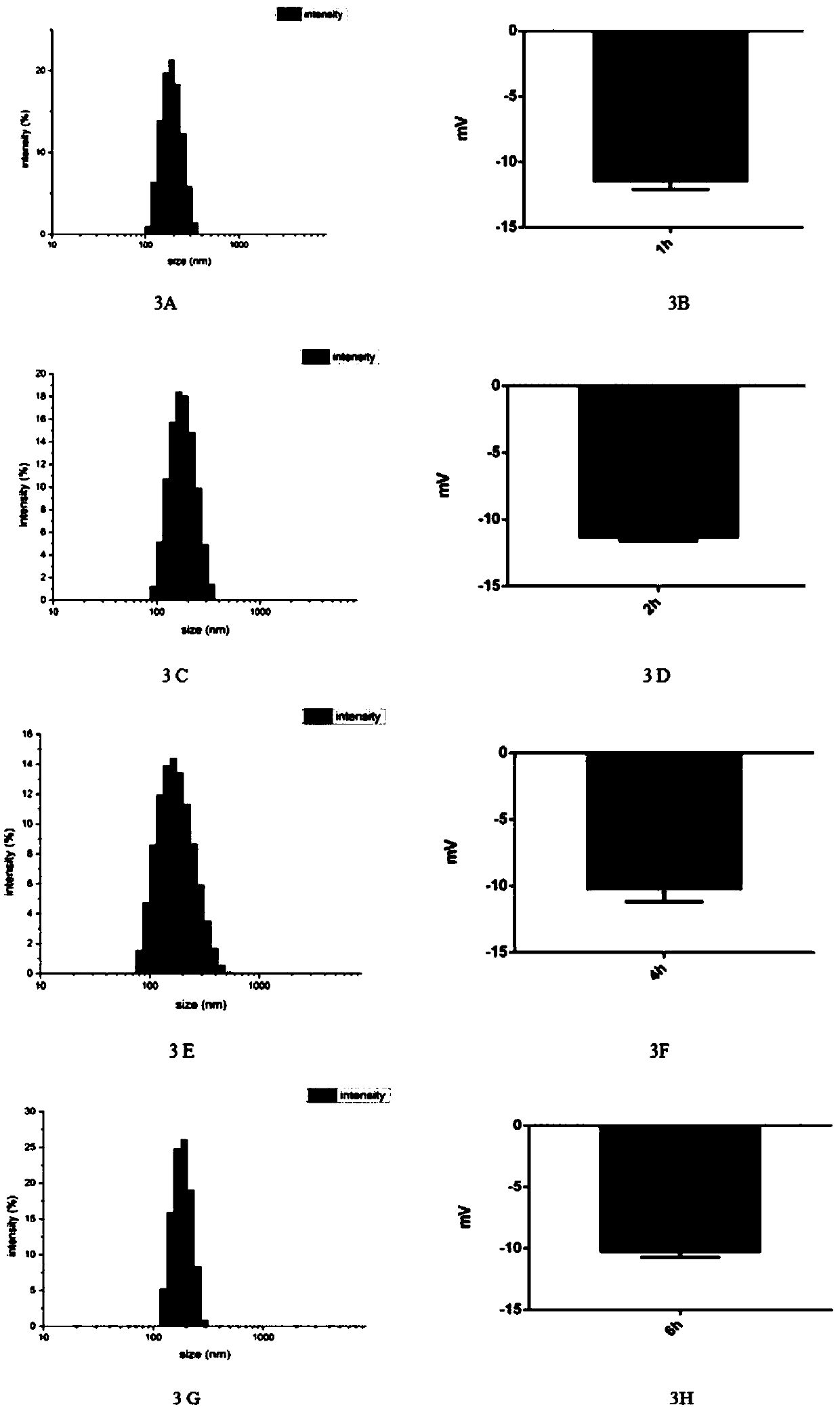
[0033] 2. After the collected lipid microbubbles were resuspended with 0.9% (g / ml) physiological saline, the resuspended lipid microbubbles were inc...
Embodiment 3
[0036] Example 3: The temperature-resistant nano-drug delivery system has a killing effect on tumor cells.
[0037] 1. Experimental materials and reagents: H22 mouse liver cancer cell line, the chemotherapeutic drug doxorubicin was purchased commercially, and milk was purchased commercially.
[0038] 2. Experimental steps:
[0039] 1) Preparation of lipid microbubbles. The preparation method is the same as in Example 1.
[0040] 2) Incubate the prepared lipid microbubbles and doxorubicin solution (1 mg / ml) at room temperature for 1 hour, then centrifuge at 20,000 g for 1 hour, and the precipitate is the drug-loaded lipid microbubbles encapsulating doxorubicin.
[0041] 3) H22 liver cancer tumor cells were cultured in RPMI medium1640 cell culture medium, and the tumor cells were planted in a 96-well plate, 1×10 4 pcs / hole.
[0042] Incubate the obtained drug-loaded lipid microbubbles with the equivalent of doxorubicin and 0.5, 2, 4ug / ml doxorubicin with the tumor cells resp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com